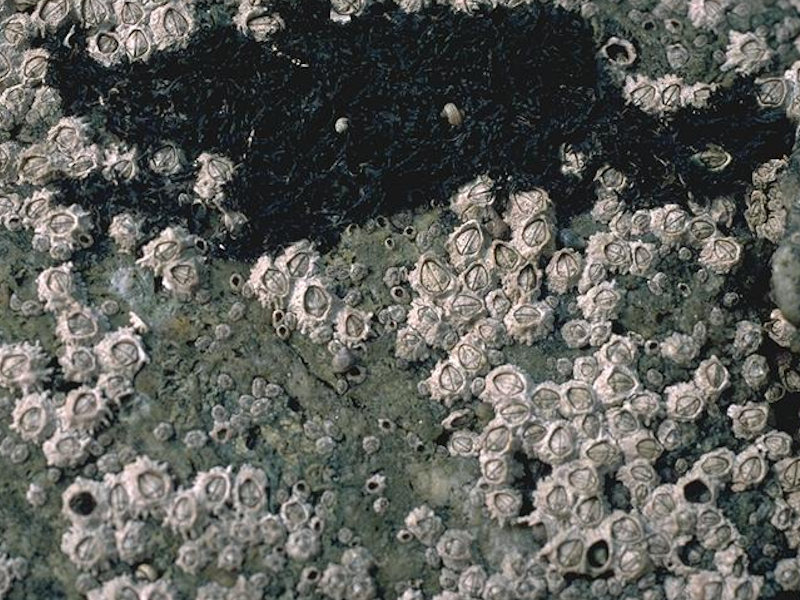
Chthamalus spp. on exposed eulittoral rock
| Researched by | Dr Heidi Tillin & Amy Watson | Refereed by | This information is not refereed |
|---|
Summary
UK and Ireland classification
Description
Very exposed to moderately exposed upper and mid eulittoral bedrock and boulders characterized by a dense community of barnacles, including Chthamalus montagui, Chthamalus stellatus and Semibalanus balanoides, and the limpet Patella vulgata. Damp cracks and crevices in the rock provide a refuge for small individuals of the mussel Mytilus edulis and the winkles Melarhaphe neritoides and Littorina saxatilis. These crevices can also be occupied by encrusting coralline algae and the anemone Actinia equina. Black patches of the lichen Verrucaria maura may be found in this zone. There is much regional variation in the distribution and zonation of Chthamalus spp. On the west coast Chthamalus spp. dominate the upper eulittoral, often forming a distinct white band above a darker band of Semibalanus balanoides in the mid eulittoral zone. Chthamalus montagui is better adapted to resist desiccation and, therefore, extends further up the shore. On some shores, particularly in the south-west, Chthamalus spp. is the dominant barnacle throughout the eulittoral zone (Cht.Cht). On other shores, particularly in the south, Lichina pygmaea can form a distinct zone (JNCC, 2015).
Depth range
Upper shore, Mid shoreAdditional information
-
Listed By
Sensitivity review
Sensitivity characteristics of the habitat and relevant characteristic species
This biotope and its two variant sub-biotopes are characterized by the presence of Chthamalus barnacles on wave exposed, rocky shores in the upper or mid to upper eulittoral. Two separate species, Chthamalus stellatus and Chthamalus montagui, are recognised (Southward, 1976). The characterizing species for this biotope are based on information from Connor et al. (2004). Connor et al. (2004) note that the distribution and shore height of Chthamalus spp. varies regionally, on west coasts Chthamalus spp. tend to be confined to the upper shore, with a distinct band of Semibalanus balanoides occurring below. In the south-west Chthamalus spp. can be dominant at all shore heights.
The variant biotope LR.HLR.MusB.Cht.Cht, can occur lower on the shore than LR.HLR.MusB.Cht.Lpyg, and may contain red and green seaweeds. Where turfs of red seaweeds are present the barnacles may be absent (Connor et al., 2004).
The variant sub-biotope LR.HLR.MusB.Cht.Lpyg is characterized by the dark brownish lichen Lichina pygmaea and the barnacles Chthamalus montagui and Chthamalus stellatus, although long-established patches of Lichina pygmaea ultimately exclude barnacles. Lichina pygmaea is a key characterizing and structuring species in this biotope as it provides habitat for the bivalve Lasaea adasoni, small littorinids and, in the south-west, the top shell Gibbula umbilicalis. It should be noted that on the north-east coast this biotope does not have Chthamalus spp., Lichina pygmaea being the most important characterizing species on these sites. Lichina pygmaea may be present in the LR.HLR.MusB.Cht.Cht biotope but does not form distinctive bands.
The limpet Patella vulgata occurs in both biotopes on the open rock surfaces, while in each sub-biotope damp crevices may shelter small Mytilus edulis, littorinids, and the anemone Actinia equina. The limpet Patella vulgata is considered a key structuring species, as its grazing can control the character of the shore by removing algae and newly settled barnacle larvae. Even a small, localised temporary absence of limpets (Southward, 1956; Southward, 1964; Hawkins, 1981; Hawkins et al., 1983), has been shown to significantly alter the biological assemblage on rocky shores. The sensitivity of this species is, therefore, specifically considered for the sensitivity assessments.
The key characterizing species considered within the sensitivity assessments are the Chthamalus barnacles (key characterizing, the limpet Patella vulgata (key characterizing and key structuring) and the lichen Lichina pygmaea (key characterizing and key structuring). The other associated common rocky shore species while contributing to diversity and function within this biotope are not considered to be key to the biotope and are therefore only generally referred to within the assessments. The hard rock or boulder substratum and wave exposure are considered key environmental factors for this biotope by providing stable attachment surfaces and by limiting the growth of macroalgae (exposure). The height on the shore is also a key factor as the species characteristic of this biotope must be able to withstand prolonged emersion. The environmental factors are considered within the sensitivity assessments where they may be altered by the pressure.
Resilience and recovery rates of habitat
Recovery of the attached characterizing species, Chthamalus spp., limpets and algal turfs will depend on recolonization by larvae. Patella vulgata is mobile, but the ability to relocate depends on the shore type and roughness (as described below). All the characterizing animal species and others that are present, such as Mytilus edulis produce pelagic larvae. As these are common, widespread species, where the footprint of the impact is relatively small, larval supply from adjacent populations should support recolonization. Where source populations are very distant due to regional impacts or habitat discontinuities, larval supply and recovery could be affected. Changes and recovery trajectories following the removal of key species are unpredictable and interactions between the key species may be positive or negative. Limpets may enhance barnacle settlement by removing algae from surfaces through grazing (Hawkins, 1983) or by depositing pedal mucus trails that attract barnacle larvae (Holmes et al., 2005), or they may crush and displace newly settled individuals (Safriel et al., 1994). Barnacles may enhance survival of small limpets by moderating environmental stresses but they may also have negative effects on recruitment by occupying space and by limiting access to grazing areas. On the wave exposed shores that this biotope occurs on, grazing may limit initial settlement of macroalgae but wave action will limit the presence of adults and larger species through, breakage and drag effects leading to loss. Mrowicki et al. (2014) found that limpet and barnacle removal allowed ephemeral and fucoid macroalgae to establish on sheltered and wave exposed shores in Ireland. Unlike the animal species macroalgae have short dispersal distances, over tens of metres (Dudgeon et al., 2001) and therefore recovery will require the presence of adults.
Recovery rates. Recolonization of Patella vulgata on rocky shores is rapid as seen by the appearance of limpet spat 6 months after the Torrey Canyon oil spill reaching peak numbers 4-5 years after the spill. However, although recolonization was rapid, the alteration to the population structure (size and age class) persisted for about 15 years because of the complex cycles of dominance (see below) involving limpets, barnacles and algae (Hawkins & Southward, 1992, Lewis & Bowman, 1975). Hence the establishment of fucoids if Patella vulgata and other grazers were absent may lead to the longer-term exclusion of this species. On rocky shores, barnacles are often quick to colonize available gaps. Bennell (1981) observed that barnacles that were removed when the surface rock was scraped off in a barge accident at Amlwch, North Wales returned to pre-accident levels within 3 years. Petraitis & Dudgeon (2005) also found that Semibalanus balanoides quickly recruited (present a year after and increasing in density) to experimentally cleared areas within the Gulf of Maine, that had previously been dominated by Ascophyllum nodosum However, barnacle densities were fairly low (on average 7.6% cover) and predation levels in smaller patches were high (Petraitis et al., 2003).
Little evidence was found for the recovery of the lichen, Lichina pygmaea, that characterizes the variant biotope LR.HLR.MusB.Cht.Lpyg. Boney (1961, 1979) studied recovery rates of Lichina pygmaea within small, experimentally cleared areas within larger lichen mats (150 cm2) and smaller semi-circular cleared patches where half of a circle was cleared. Boney, (1979) found that no recovery had occurred within 24 years in the smaller, patches although in one small cleared circle some regrowth was appearing at the edges. Within the mats, the cleared areas expanded due to the dieback of lichen, eleven to twelve years after clearance, the distribution of the plants within the larger Lichina pygmaea mats had completely altered and the original experimental site was unrecognisable with complete loss of lichen and no recolonization from surrounding mats. It should be noted that some natural reduction of adjacent patches was also observed and some variation in extent occurs naturally over time, although the loss outside of the experimental areas was slight and localised (Boney, 1979). Cleared areas were first colonized by green algae and barnacles with subsequent denser growths of Fucus spiralis close to the patches.
Life histories and reproduction. The lifespan of Chthamalus spp. is 10+ years (Mieszkowska et al., 2014). Sexual maturity can be reached in the first year and a number of broods may be produced each year. Burrows et al. (1992) found that the number of eggs per brood of Chthamalus stellatus ranged between 1,274 - 3,391 in Britain, depending on body size and weight. Shore height affects a number of life history parameters, growth is more rapid and the mortality rate is greater lower down on the shore (Southward & Crisp, 1950). Towards the northern limits of distribution annual recruitment is low (Kendall & Bedford, 1987) and they have an increased longevity (Lewis, 1964). Burrows et al. (1992) found that the fecundity generally increased with lower shore levels colonized, with estimations of 1-2 broods per year at high shore levels, 2 to over three at mid shore levels, and over 2 to over 4 at low shore levels.
Southward (1978) suggested that Chthamalus montagui breeds one to two months later than Chthamalus stellatus. However, Crisp et al. (1981) found little difference in SW Britain, with the main breeding peak in June and August (O'Riordan et al., 1995). Throughout the breeding season, most individuals produce several broods (Burrows et al., 1992; O'Riordan et al., 1992), with a small percentage of the population remaining reproductively active throughout the year (O'Riordan et al., 1995; Barnes, 1989).
In northern England, Patella vulgata reached sexual maturity in their second year (Blackmore, 1969) and thereafter reproduce annually. Limpets may change sex during their lifetime, with younger animals being male and older animals tending to be female (Blackmore, 1969). In Robin Hood’s Bay, Lewis & Bowman (1975) observed spawning of Patella vulgata in the Autumn, with spatfall occurring in winter when desiccation pressures were lower. The rate and density of colonization are affected by the presence of other species. Lewis & Bowman (1975), observed that mussels promote settlement of Patella vulgata. The settlement was also higher amongst barnacles and light coverings of algae. Dense coverings of mussels and fucoids, however, inhibit settlement through competition for space or prevention of settlement.
Local environmental conditions, including surface roughness (Hills & Thomason, 1998), wind direction (Barnes, 1956), shore height, wave exposure (Bertness et al., 1991) and tidal currents (Leonard et al., 1998) have been identified, among other factors, as factors affecting settlement of Semibalanus balanoides. Biological factors such as larval supply, competition for space, the presence of adult barnacles (Prendergast et al., 2009) and the presence of species that facilitate or inhibit settlement (Kendall et al., 1985, Jenkins et al., 1999) also play a role in recruitment. Mortality of juveniles can be high but highly variable, with up to 90% of Semibalanus balanoides dying within ten days (Kendall et al., 1985). Presumably, these factors would also influence the transport, supply and settlement of Chthamaus spp.
Resilience assessment. No evidence for recovery rates was found specifically for the biotopes and sub-biotopes (LR.HLR.MusB.Cht.Cht , LR.HLR.MusB.Cht.Lpyg). The evidence suggests that the size of the footprint of an impact and the magnitude will influence the recovery rates by mediating settlement and post-settlement recruitment. Barnacles are attracted to settle in the presence of adults of the same species (Prendergast et al., 2009) so that the presence of adults will facilitate recovery. In the barnacle dominated biotope LR.HLR.MusB.Cht.Cht, resilience is assessed as ‘High’ (within 2 years) where resistance is ‘High’ (no significant impact) or 'Medium' (<25% of characteristic biotope removed). A resistance of 'Medium' assumes that either a large proportion of the biotope is unimpacted or that the entire biotope is impacted but only a proportion of the characterizing species are removed, with unimpacted areas or individuals supporting recovery. Resilience is assessed as ‘Medium’ (2-10 years) where resilience is ‘None’ or ‘Low’ as recruitment may be episodic and the age structure of the limpet population will require more time to recover.
However in the mixed barnacle, lichen biotope LR.HLR.MusB.Cht.Lpyg, resilience is assessed as ‘Medium’ where resistance is ‘High’ (no significant impact) or 'Medium' (<25% of characteristic biotope removed) as Lichina pygmaea may take many years to recover and that cleared patches in areas of growth may expand. Hence, resilience is assessed as ‘Low' to 'Very low’ where resistance is ‘Low’ or ‘None’, although confidence in the applicability and degree of concordance is 'Low' as further supporting evidence confirming this finding was not found.
Note: the resilience and the ability to recover from human induced pressures is a combination of the environmental conditions of the site, the frequency (repeated disturbances versus a one-off event) and the intensity of the disturbance. Recovery of impacted populations will always be mediated by stochastic events and processes acting over different scales including, but not limited to, local habitat conditions, further impacts and processes such as larval-supply and recruitment between populations. Full recovery is defined as the return to the state of the habitat that existed prior to impact. This does not necessarily mean that every component species has returned to its prior condition, abundance or extent but that the relevant functional components are present and the habitat is structurally and functionally recognisable as the initial habitat of interest. It should be noted that the recovery rates are only indicative of the recovery potential.
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidenceSpecies found in the intertidal are exposed to extremes of high and low air temperatures during periods of emersion. They must also be able to cope with sharp temperature fluctuations over a short period of time during the tidal cycle. In winter air temperatures are colder than the sea, conversely in summer air temperatures are much warmer than the sea. Intertidal species are therefore likely to have a degree of resistance to temperature changes, with species found higher on the shore able to tolerate a greater thermal range (Davenport & Davenport, 2005). This biotope may contain a mix of Chthamalus and Semibalanus balanoides, barnacles, particularly at mid-shore levels. Increased temperatures are likely to favour chthamalid barnacles rather than Semibalanus balanoides (Southward et al. 1995). Chthamalus montagui and Chthamalus stellatus are warm water species, with a northern limit of distribution in Britain so are likely to be tolerant of long-term increases in temperature. The range of Chthamalus stellatus and Chthamalus montagui has been extending northwards due to increasing temperatures. Breeding of Chthamalus stellatus in France occurs in April (Barnes, 1992), and correlates with mean air and sea temperatures of 11 - 12°C, and maximum temperatures of 14°C. Barnes (1992) found that at an upper temperature limit of 20 - 21°C in the sea and 24 - 25°C in the air reproductive activity decreased. Chthamalus suffers a failure of fertilization at temperatures of 9°C and below (Patel & Crisp, 1960), its lower critical temperature for feeding activity is 4.6°C (Southward, 1955). Semibalanus balanoides out-competes Chthamalus species for space, but recruitment declines and failures of Semibalanus balanoides in response to warmer temperatures benefit Chthamalus species by allowing them to persist and recruit (Mieszkowska et al., 2014). The limpet Patella vulgata is 'northern' species with their range extending from Portugal or Northern Spain to the Arctic circle. Populations in the southern part of England are therefore relatively close to the southern edge of their geographic range. The body temperature of Patella vulgata can exceed 36°C in the field, (Davies, 1970), adults become non-responsive at 37-38°C and die at temperatures of 42°C (Evans, 1948). Juvenile tolerance of warm air temperatures and desiccation may be lower than adults. Juveniles require damp areas of rock (Lewis & Bowmna, 1975) and the bare rock surfaces typical of this biotope, present a harsher habitat than the associated crevices and cracks. Long-term studies in southern England suggest that Patella vulgata has become scarcer following warmer summers, while Patella depressa, a species with a more southerly distribution has increased in abundance (Southward et al., 1995). Increased temperatures may alter spawning cues and reproduction success in Patella vulgata populations. Observations suggest that spawning is initiated in autumn storms with greater wave action when seawater temperatures drop below 12°C (Bowman 1985, Bowman & Lewis, 1986, LeQuesne, 2005). In northern Portugal, warming seas appear to be linked to a shortening of the reproductive period and the lack of multiple spawning events in Patella vulgata and other northern species (Ribeiro et al., 2009). Most of the other species within the biotope are eurythermal (e.g. Nucella lapillus and Mytilus edulis) and are hardy intertidal species that tolerate long periods of exposure to the air and consequently wide variations in temperature. In addition, most species, including Lichina pygmaea (Guiry & Guiry, 2015) are distributed to the north and south of the British Isles and unlikely to be adversely affected by long-term temperature changes at the benchmark level. Corallina officinalis, however, experienced severe damage during the unusually hot summer of 1983 (Hawkins & Hartnoll, 1985). Sensitivity assessment. Adult Chthamalus spp. and Patella vulgata are considered likely to be able to tolerate an acute or chronic change. Resistance is, therefore, assessed as ‘High’ and resilience as ‘High’ (by default). Hence, this biotope is considered to be ‘Not sensitive’ at the pressure benchmark. Sensitivity to longer-term, broad-scale perturbations such as increased temperatures from climate change would, however, be greater, based on the extent of impact and the reduction in larval supply. | HighHelp | HighHelp | Not sensitiveHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceMany intertidal species are tolerant of freezing conditions as they are exposed to extremes of low air temperatures during periods of emersion. They must also be able to cope with sharp temperature fluctuations over a short period of time during the tidal cycle. In winter air temperatures are colder than the sea, conversely in summer air temperatures are much warmer than the sea. Species that occur in the intertidal are therefore generally adapted to tolerate a range of temperatures, with the width of the thermal niche positively correlated with the height of the shore that the animal usually occurs at (Davenport & Davenport, 2005). Chthamalus stellatus and Chthamalus montagui are ‘southern’ species and their range has been extending northwards due to increasing temperatures. Chthamalus suffers a failure of fertilization at temperatures of 9°C and below (Patel and Crisp, 1960) its lower critical temperature for feeding activity is 4.6°C (Southward, 1955). The cold winter of 2009-10 in France led to recruitment failure in Chthamalus species (Wethey et al., 2011). The barnacle Semibalanus balanoides has a greater tolerance for cooler temperatures and a decrease in temperature may enhance recruitment success and replacement of Chthamalus spp. The tolerance of Semibalanus balanoides collected in the winter (and thus acclimated to lower temperatures) to low temperatures was tested in the laboratory. The median lower lethal temperature tolerance was -14.6°C (Davenport & Davenport, 2005). A decrease in temperature at the pressure benchmark is therefore unlikely to negatively affect this species. The distribution of the key characterizing species, Patella vulgata is 'northern', extending from Northern Spain to the Arctic circle where they are subject to lower temperatures than in the UK. Adults are largely unaffected by short periods of extreme cold. Ekaratne & Crisp (1984) found adult limpets continuing to grow over winter when temperatures fell to -6°C and stopped only by still more severe weather. However, loss of adhesion after exposure to -13°C has been observed with limpets falling off rocks and therefore becoming easy prey to crabs or birds (Fretter & Graham, 1994) and in the very cold winter of 1962-3 when temperatures repeatedly fell below 0°C over a period of 2 months large numbers of Patella vulgata were found dead (Crisp, 1964). Periods of frost may also kill juvenile Patella vulgata, resulting in recruitment failures in some years (Bowman & Lewis, 1977). The associated species Mytilus edulis is a eurytopic species found in a wide temperature range and in areas which frequently experience freezing conditions and are vulnerable to ice scour (Seed & Suchanek 1992). After acclimation of individuals of Mytilus edulis to 18°C, Kittner & Riisgaard (2005) observed that the filtrations rates were at their maximum between 8.3 and 20°C and below this at 6°C the mussels closed their valves. However, after being acclimated at 11°C for five days, the mussels maintained the high filtration rates down to 4 °C. Hence, given time, mussels can acclimatise and shift their temperature tolerance. Filtration in Mytilus edulis was observed to continue down to -1°C, with high absorption efficiencies (53-81 %) (Loo, 1992). No specific evidence was found for thermal tolerance of the lichen Lichina pygmaea that is a key characterizing species for the variant biotope LR.HLR.MusB.Cht.Lpyg, however, this species is distributed to the north and south of the British Isles (Guiry & Guiry, 2015) and is therefore considered to be tolerant of changes in temperature at the pressure benchmark. Sensitivity assessment. Based on evidence for the wide temperature tolerance range of Patella vulgata it is concluded that the acute and chronic decreases in temperature described by the benchmark would have limited effect. Adult Chthamalus spp. are considered to tolerate a wide range of temperatures and to be unaffected by a chronic decrease in temperature at the pressure benchmark. An acute change may impact the reproductive success or lead to adult mortalities during colder winters when threshold tolerances were breached. However, due to the persistence of Chthamalus spp. around the UK, changes at the pressure benchmarks are considered unlikely to lead to changes in the adult population within the biotope. Based on the key characterizing species and Mytilus edulis this biotope is considered to have ‘High’ resistance and ‘High' resilience (by default) to this pressure and is, therefore, considered to be ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceBiotopes found in the intertidal will naturally experience fluctuations in salinity where evaporation increases salinity and inputs of rainwater expose individuals to freshwater. Species found in the intertidal are therefore likely to have some form of behavioural or physiological adaptations to changes in salinity. This biotope is found in full (30-35 ppt) salinity (Connor et al., 2004). Barnes & Barnes (1974) found that larvae from six barnacle species including Chthamalus stellatus and Semibalanus (as Balanus) balanoides, completed their development to nauplii larvae at salinities between 20-40% and some embryos exposed at later development stages could survive at higher and lower salinities. No evidence was found to assess the sensitivity of Lichina pygmaea to changes in salinity at the pressure benchmark and this pressure is not assessed. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceBiotopes found in the intertidal will naturally experience fluctuations in salinity where evaporation increases salinity and inputs of rainwater expose individuals to freshwater. Species found in the intertidal are therefore likely to have some form of behavioural or physiological adaptations to changes in salinity. As this biotope is present in full salinity, the assessed change at the pressure benchmark is a reduction in salinity to a variable regime (18-35 ppt). The key characterizing and structuring species Patella vulgata can tolerate varying salinities and its distribution extends into the mouths of estuaries surviving in salinities down to about 20 psu. However, growth and reproduction may be impaired in reduced salinity. Little et al. (1991), for example, observed reduced levels of activity in limpets after heavy rainfall and in the laboratory activity completely stopped at 12psu. The species can endure periods of low salinity. It was found to die only when the salinity was reduced to 3-1 psu (Fretter & Graham, 1994). In experiments where freshwater was trickled over the shell Arnold (1957) observed limpets withdrawing and clamping the shell onto the substratum. There appears to be an increasing tolerance of low salinities from the lower to the upper limit of distribution of the species on the shore (Fretter & Graham, 1994). Barnes & Barnes (1974) found that larvae from six barnacle species including Chthamalus stellatus and Semibalanus (as Balanus) balanoides, completed their development to nauplii larvae at salinities between 20-40% (some embryos exposed at later development stages could survive at higher and lower salinities). Barnes & Barnes (1965) found that in high suspended solids and low salinity there was a decrease in the number of eggs per brood of Chthamalus stellatus / Chthamalus montagui. If salinities decrease below 21 psu all cirral activity of barnacles normally associated with full salinity waters, ceases (Foster, 1971). Semibalanus balanoides are tolerant of a wide range of salinity and can survive periodic emersion in freshwater, e.g. from rainfall or freshwater runoff, by closing their opercular valves (Foster, 1971b). They can also withstand large changes in salinity over moderately long periods of time by falling into a "salt sleep" and can be found on shores (example from Sweden) with large fluctuations in salinity around a mean of 24 (Jenkins et al., 2001). Similarly, most of the associated species (e.g. Mytilus edulis) are found in a wide range of salinities and are probably tolerant of 'variable' or 'reduced' salinity, although no evidence was found for the lichen Lichina pygmaea. The intertidal interstitial invertebrates and epifauna probably experience short-term fluctuating salinities, with reduced salinities due to rainfall and freshwater runoff when emersed. Prolonged reduction in salinity, e.g. from full to reduced due to e.g. freshwater runoff, is likely to reduce the species richness of the biotope due to loss of less tolerant red algae and some intolerant invertebrates. However, the dominant species will probably survive and the integrity of the biotope is likely to be little affected. Areas of freshwater runoff in the intertidal promote the growth of ephemeral greens, probably due to their tolerance of low salinities and inhibition of grazing invertebrates. Sensitivity assessment. Based on reported distributions and the results of experiments to assess salinity tolerance thresholds and behavioural and physiological responses in Patella vulgata, Chthamalus spp. and other characterizing species it is considered that adults of this species would be able to tolerate a change to a variable salinity regime for a year. However, if salinities were around 18 ppt for prolonged periods some decreases in species abundance would be expected. Larvae may be more sensitive than adult life stages and recruitment may be decreased. Over a period of time longer than a year this would lead to the replacement of the biotope by species more typical of estuarine conditions such as the non-native barnacle Austrominius (formerly Elminius) modestus. Resistance is assessed as 'Medium' for some changes in population abundance, and resilience is assessed as 'High', the biotope is therefore considered to have 'Low' sensitivity, although it should be noted that sensitivity to prolonged exposure to salinities around 18 ppt may be greater. | MediumHelp | HighHelp | LowHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidenceThe biotope is characteristic of very exposed to moderately wave exposed conditions where water movement from wave action will exceed the strength of any possible tidal flow (Connor et al., 2004). The evidence is presented for the tolerance of the key characterizing species, Semibalanus balanoides and Patella vulgata to changes in water flow. However, it should be noted that wave action is more significant as an environmental factor than water flow for this biotope. Growth and reproduction of Semibalanus balanoides are influenced by food supply and water velocity (Bertness et al., 1991). Laboratory experiments demonstrate that barnacle feeding behaviour alters over different flow rates but that barnacles can feed at a variety of flow speeds (Sanford et al., 1994). Flow tank experiments using velocities of 0.03, 0.07 and 0.2 m/s showed that a higher proportion of barnacles fed at higher flow rates (Sanford et al., 1994). Feeding was passive, meaning the cirri were held out to the flow to catch particles; although active beating of the cirri to generate feeding currents occurs in still water (Crisp & Southward, 1961). Field observations at sites in southern New England (USA) that experience a number of different measured flow speeds, found that Semibalanus balanoides from all sites responded quickly to higher flow speeds, with a higher proportion of individuals feeding when current speeds were higher. Barnacles were present at a range of sites, varying from sheltered sites with lower flow rates (maximum observed flow rates <0.06- 0.1 m/s), a bay site with higher flow rates (maximum observed flows 0.2-0.3 m/s) and open coast sites (maximum observed flows 0.2-0.4 m/s). Recruitment was higher at the site with flow rates of 0.2-0.3 m/s (although this may be influenced by supply) and at higher flow microhabitats within all sites. Both laboratory and field observations indicate that flow is an important factor with effects on feeding, growth and recruitment in Semibalanus balanoides (Sanford et al., 1994; Leonard et al., 1998), however, the results suggest that flow is not a limiting factor determining the overall distribution of barnacles as they can adapt to a variety of flow speeds. Patella vulgata inhabits a range of tidal conditions and is, therefore, likely to tolerate a change in water flow rate. The streamlined profile of limpet shells is of importance in increasing their tolerance of water movement, and this is undoubtedly one factor in determining the different shape of limpets at different exposures. With increasing exposure to wave action, the shell develops into a low profile reducing the risk of being swept away. The strong muscular foot and a thin film of mucus between the foot and the rock enables Patella vulgata to grip very strongly to the substratum (Fretter & Graham, 1994). The ability of limpets to resist accelerating, as distinct from constant currents, may set a limit to the kind of habitat they can occupy and limit the size to which they can grow. Sensitivity assessment. The biotope is characteristic of very exposed to moderately wave exposed conditions where water movement from wave action will greatly exceed the strength of any possible tidal flow. The available evidence indicates that the characterizing species Patella vulgata and Semibalanus balanoides are able to adapt to high flow rates and the biotope is therefore considered to be 'Not sensitive' to an increase in water flow. A decrease in water flow may have some effects on recruitment and growth, but this is not considered to be lethal at the pressure benchmark and resistance is therefore assessed as 'High' and resilience as 'High' by default, so that the biotope is considered to be 'Not sensitive'. A decrease in water flow, exceeding the pressure benchmark, coupled with a decrease in wave action, may, however, alter the character of the biotope to LR.MLR.MusF.MytFR or LR.MLR.MusF.MytFves, where brown seaweeds were able to proliferate at mid-shore levels and the edible periwinkle Littorina littorea was able to colonize. | HighHelp | HighHelp | Not sensitiveHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidenceEmergence regime is a key factor in structuring this (and other) intertidal biotopes. Increased emergence may reduce habitat suitability for characterizing species through greater exposure to desiccation and reduced feeding opportunities for the barnacles which feed when immersed. Changes in emergence may, therefore, lead to species replacement and the development of a biotope, more typical of the changed shore level may develop. Both the variant sub-biotopes are typically found below a zone of the black lichen, Verrucaria maura and above a Semibalanus balanoides band with mussels or limpets (Connor et al., 2004). Some regional variation occurs in the distribution of Chthamalus on the shore and the vertical zonation will be affected by wave splash and shore steepness. On shores on the West coast of Scotland, Chthamalus stellatus and Chthamalus montagui are restricted to high shores as Semibalanus balanoides, the northern species is competitively superior at these latitudes (Connell, 1961a,b). Further south in the UK, the two genera coexist in the mid-shore (Crisp et al., 1981). Increased emergence would reduce the feeding time for attached suspension feeders within the biotope and the increased desiccation. It is likely that the distribution of Chthamalus stellatus will move further up the shore, with no noticeable difference in the range. Chthamalus stellatus / Chthamalus montagui are very tolerant of high periods of emersion, yet Patel & Crisp (1960) found that when barnacles which were brooding eggs were kept out of the water, a second batch of eggs was not produced. Increased desiccation may also have a negative impact the lichen Lichina pygmaea, which is a key characterizing species within the variant biotope LR.HLR.MusB.Cht.Lpyg, and which prefers areas of rock that retain some moisture (Boney, 1961; Kronberg, 1988). Decreased emergence is likely to lead to the habitat the biotope is found in becoming more suitable for the lower shore species generally found below the biotope, leading to replacement. Adults of Chthamalus stellatus can survive permanent submersion (Barnes, 1953). However, competition between Semibalanus balanoides is likely to play an important role in the changes in species distribution. Semibalanus balanoides is less tolerant of desiccation stress than Chthamalus barnacles species but is considered to out-compete Chthamalus spp. in the mid and lower shore. If wave splash reached higher up the shore following changes in emergence, new areas of the shore may become suitable for lichens, including the characterizing Lichina pygmaea. However, it should be noted that recolonization and growth are very slow within these species and altered competitive dynamics between species may prevent colonisation of new areas by Lichina pygmaea within the footprint of the impact through competition and exclusion by other species, particularly fucoids. The mobile species present within the biotope; Patella vulgata and the littorinids would be able to relocate to preferred shore levels. Sensitivity assessment. As emergence is a key factor structuring the distribution of animals on the shore, resistance to a change in emergence (increase or decrease) is assessed as ‘Low’. Resilience is assessed as ‘Medium’ for the variant biotope LR.HLR.MusB.Cht.Cht and 'Low' to 'Very low' for the variant biotope LR.HLR.MusB.Cht.Lpyg and sensitivity is therefore assessed as 'Medium' or 'High' respectively. The higher sensitivity assessment for LR.HLR.MusB.Cht.Lpyg is recorded. | LowHelp | LowHelp | HighHelp |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidenceThis biotope is recorded from locations that range from very wave exposed, moderately exposed to wave exposed (Connor et al., 2004). The degree of wave exposure influences wave height, as in more exposed areas with a long fetch, waves would be predicted to be higher. As this biotope occurs across three wave exposure categories, this was therefore considered to indicate, by proxy, that biotopes in the middle of the wave exposure range would tolerate either an increase or decrease in significant wave height at the pressure benchmark. An increase or decrease in wave action, exceeding the pressure benchmark, may, however, alter the character of the biotope. In Britain and Ireland, the recruitment of Chthamalus stellatus and Chthamalus montagui overlaps (Crisp et al., 1981) but some trends in abundance have been observed. Chthamalus montagui increases in abundance in sheltered locations and towards the high-water neap-tide level on all shores where chthamalid species occur. Chthamalus stellatus, with increasing exposure, extends upwards into the Chthamalus montagui zone (Delaney et al., 2003). Changes in wave height may, therefore, change the ranges of the two species but this would not alter the character of the biotope. The edible periwinkle Littorina littorea may also colonize suitable areas following a decrease in wave exposure. A decrease in wave exposure may ultimately reduce Patella vulgata abundance because the species does not favour thick algal cover that is often present on very sheltered shores. Alternatively, an increase in significant wave height, linked to increased exposure, may result in population changes with fewer barnacles present and with the limpet Patella ulyssiponensis present, or present in greater numbers, rather than Patella vulgata (Thompson, 1980). Observations in Wales found that lichens form a thick band at the upper levels of extremely wave exposed and very wave exposed shores and Lichina pygmaea is common (1-20% cover) on shores that are very exposed to wave action (Ballantine, 1961). It is also common or abundant (more than 20% cover at some levels) on exposed shores and also occurs at lower abundances on semi-exposed and fairly sheltered shores but is absent from sheltered and very sheltered shores (Ballantine, 1961). Naylor, (1930) also found that Lichina pygmaea was absent from more sheltered shores in Plymouth Sound and was present where wave exposure was greater. In sheltered shores and those with flatter surfaces competition from fucoids excludes the lichen (Naylor, 1930). Sensitivity assessment. The natural wave exposure range of this biotope is considered to exceed changes at the pressure benchmark and this biotope is considered to have 'High' resistance and 'High' resilience (by default), to this pressure (at the benchmark) and is assessed as 'Not sensitive'. | HighHelp | HighHelp | Not sensitiveHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Contamination at levels greater than the benchmark may impact this biotope. However, barnacles, may tolerate a fairly high level of heavy metals in nature, for example, they possess metal detoxification mechanisms and are found in Dulas Bay, Anglesey, where copper reaches concentrations of 24.5 µg/l, due to acid mine waste (Foster et al., 1978; Rainbow, 1984). Bryan (1984) suggested that gastropods are also rather tolerant of heavy metals. In the Fal estuary Patella vulgata occurs at, or just outside, Restronguet Point at the end of the creek where metal concentrations are in the order: Zinc (Zn) 100-2000 µg/l, copper (Cu) 10-100 µg/l and cadmium (Cd) 0.25-5 µg/l (Bryan & Gibbs, 1983). However, in the laboratory, Patella vulgata was found to be intolerant of small changes in environmental concentrations of Cd and Zn by Davies (1992). At concentrations of 10µg/l pedal mucus, production and levels of activity were both reduced, indicating a physiological response to metal concentrations. Exposure to Cu at a concentration of 100 µg/l for one week resulted in progressive brachycardia (slowing of the heart beat) and the death of limpets. Zn at a concentration of 5500 µg/l produced the same effect (Marchan et al.,1999). | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Hydrocarbon contamination, at levels greater than the benchmark, e.g. from spills of fresh crude oil or petroleum products, may cause significant loss of component species in the biotope, through impacts on individual species viability or mortality, and resultant effects on the structure of the community (Suchanek, 1993; Raffaelli & Hawkins, 1999).In areas of moderate oil deposit, up to about 1/2cm thick, on rocks after the Torrey Canyon oil spill, limpets had survived unscathed over a month after the event and feeding continued even though a coating of oil smothered their food source of algae and diatoms (Smith, 1968). Limpets can ingest thick oil and pass it through their gut. However, thick layers of oil smothering individuals will interfere with respiration and spoil normal food supplies for Patella vulgata. Limpets are unable to remain closed off from the environment for very long, the adductor muscles relax occasionally, lifting the shell very slightly. After the Braer oil spill, in common with many other oil spills, the major impact in the intertidal zone was on the population of limpets and other grazers. In West Angle Bay, where fresh oil from the Sea Empress tanker reached rocky shores within one day of the spill, limpet mortality was 90% (Glegg et al., 1999). Thus Patella vulgata has a higher intolerance to fresh oil which has a high component of volatile hydrocarbons remaining. A significant reduction in the density of juvenile limpets was also observed at all sites known to have been oiled by the Sea Empress spill (Moore, 1997). In long-term studies into the environmental effects of oil refinery effluent discharged into Littlewick Bay, Milford Haven, the number of limpets, usually found in substantial numbers on this type of shore, were considerably reduced in abundance on areas close to the discharge (Petpiroon & Dicks, 1982). In particular, only large individuals were found close to the outfall point and juveniles were completely absent, suggesting that observed changes in abundance resulted from effluent effects on larval stages rather than upon adults directly. Littoral barnacles (e.g. Semibalanus balanoides) have a high resistance to oil (Holt et al., 1995) but may suffer some mortality due to the smothering effects of thick oil (Smith, 1968). However, laboratory studies of the effects of oil and dispersants on several red algae species (Grandy, 1984 cited in Holt et al. 1995) concluded that they were all sensitive to oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. O'Brien & Dixon (1976) suggested that red algae were the most sensitive group of algae to oil or dispersant contamination. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed but evidence is presented where available. Synthetic compound contamination, at levels greater than the benchmark, is likely to have a variety of effects depending the specific nature of the contaminant and the species group(s) affected. Barnacles have a low resilience to chemicals such as dispersants, dependant on the concentration and type of chemical involved (Holt et al., 1995). Hoare & Hiscock (1974) reported that the limpet Patella vulgata was excluded from sites within 100-150 m of the discharge of acidified, halogenated effluent in Amlwch Bay. Limpets are also extremely intolerant of aromatic solvent based dispersants used in oil spill clean-up. During the clean-up response to the Torrey Canyon oil spill nearly all the limpets were killed in areas close to dispersant spraying. Viscous oil will not be readily drawn in under the edge of the shell by ciliary currents in the mantle cavity, whereas detergent, alone or diluted in seawater, would creep in much more readily and be liable to kill the limpet (Smith, 1968). A concentration of 5ppm killed half the limpets tested in 24 hours (Southward & Southward, 1978; Hawkins & Southward, 1992). Acidified seawater affects the motility of Patella vulgata. At a pH of 5.5 motility was reduced whilst submerged but individuals recovered when returned to normal seawater. At a pH of 2.5 total inhibition of movement occurred and when returned to normal seawater half had died (Bonner et al., 1993). Reduced motility reduces the time for foraging and may result in decreased survival of individuals. Acidified seawater can also change the shell composition which will lead to a decrease in its protective nature and hence survival (Bonner et al., 1993). Short periods (48 hours) are unlikely to have much effect on a population but long periods (1 year) may cause reduced grazing and an increase in algal growth. However, seawater is unlikely to reach pH 2.5, therefore, intolerance to slight changes in pH will be low. Gastropod molluscs are known to be intolerant of endocrine disruption from synthetic chemicals such as tri-butyl tin (Cole et al., 1999). However, no information on the specific effects of tri-butyl tin on Patella vulgata was found. Red algae are probably intolerant of chemical contamination. O'Brien & Dixon (1976) suggested that red algae were the most sensitive group of algae to oil contamination, although the filamentous forms were the most sensitive. Laboratory studies of the effects of oil and dispersants on several red algae species, including Palmaria palmata (Grandy, 1984 cited in Holt et al., 1995) concluded that they were all sensitive to oil/ dispersant mixtures, with little differences between adults, sporelings, diploid or haploid life stages. Cole et al. (1999) suggested that herbicides, such as simazine and atrazine were very toxic to macrophytes. In addition, Hoare & Hiscock (1974) noted that almost all red algae were excluded from Amlwch Bay, Anglesey by acidified halogenated effluent discharge. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceNo evidence. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceThis pressure is Not assessed. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceAn oxygen concentration at the level of the benchmark, 2 mg/l, is thought likely to cause adverse effects in marine organisms. In laboratory experiments, a reduction in the oxygen tension of seawater from 148 mm Hg (air saturated seawater) to 50 mmHg rapidly resulted in reduced heart rate in limpets of the genus Patella (Marshall & McQuaid, 1993). Heartbeat rate returned to normal in oxygenated water within two hours. Limpets can survive for a short time in anoxic seawater; Grenon & Walker (1981) found that in oxygen-free water limpets could survive up to 36 hours, although Marshall & McQuaid (1989) found a lower tolerance for Patella granularis, which survived up to 11 hours in anoxic water. Therefore, some individuals may survive for one week at an oxygen concentration of 2 mg/l. Exposure would be mediated by the position of the biotope in the upper to mid-shore as Patella vulgata is able to respire in the air and would only be exposed to low oxygen in the water column intermittently during periods of tidal immersion. In addition, in areas of wave exposure and moderately strong current flow low oxygen levels in the water are unlikely to persist for very long. Barnacles seem to have a high tolerance of anaerobic conditions. Chthamalus stellatus / Chthamalus montagui have, for example, been shown to be relatively unaffected by smothering by oil. Monterosso (1930) showed experimentally that the species can survive complete smothering by petroleum jelly for approximately two months, by respiring anaerobically. Complete smothering caused by the Torrey Canyon oil spill yielded similar results; a few Semibalanus balanoides died, yet Chthamalus stellatus / Chthamalus montagui seemed unaffected (Smith, 1968). Semibalanus balanoides can respire anaerobically, so they can tolerate some reduction in oxygen concentration (Newell, 1979). When placed in wet nitrogen, where oxygen stress is maximal and desiccation stress is low, Semibalanus balanoides have a mean survival time of five days (Barnes et al., 1963). Sensitivity assessment. The characterizing species Patella vulgata and Semibalanus balanoides are considered to be ‘Not Sensitive’ to deoxygenation at the pressure benchmark. Resistance is, therefore, assessed as ‘High’ and resilience as ‘High’ (no effect to recover from). In addition, as this biotope occurs in the intertidal, emergence will mitigate the effects of hypoxic surface waters as will the exposure to wave action and water flows and the biotope is considered to be 'Not sensitive' to this pressure. | HighHelp | HighHelp | Not sensitiveHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceNo direct evidence was found to assess this pressure. A slight increase in nutrient levels could be beneficial for barnacles and mussels by promoting the growth of phytoplankton levels and therefore increasing zooplankton levels. Limpets and other grazers would also benefit from the increased growth of benthic microalgae. However, Holt et al. (1995) predict that smothering of barnacles by ephemeral green algae is a possibility under eutrophic conditions and if nutrient loading is excessive this can have a detrimental effect on algal productivity and hence limpet growth. Sensitivity assessment. The pressure benchmark is set at a level that is relatively protective and based on the evidence and considerations outlined above the biological assemblage is considered to be 'Not sensitive' at the pressure benchmark. Resistance and resilience are therefore assessed as 'High'. | HighHelp | HighHelp | Not sensitiveHelp |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceOrganic enrichment may lead to eutrophication with adverse environmental effects including deoxygenation, algal blooms and changes in community structure (see nutrient enrichment and de-oxygenation). The biotopes occur in tide-swept or wave exposed areas (Connor et al., 2004) preventing a build-up of organic matter so that the biotope is considered to have a low risk of organic enrichment at the pressure benchmark. Little evidence was found to support this assessment, Cabral-Oliveira et al. (2014), found that filter feeders such as Mytilus sp. and the barnacle Chthamalus montagui, were more abundant at sites closer to a sewage treatment works, as they could utilise the organic matter inputs as food. On the same shores, higher abundances of juvenile Patella sp. and lower abundances of adults were found closer to sewage inputs, Cabral-Oliveira et al., (2014) suggested the structure of these populations was due to increased competition closer to the sewage outfalls. Sensitivity assessment. Little empirical evidence was found to support an assessment of the barnacles and Patella vulgata within this biotope. As organic matter particles in suspension or re-suspended could potentially be utilised as a food resource by filter feeders present within the biotope (Cabral-Oliveira et al., 2014) with excess likely to be rapidly removed by wave action, overall resistance of the biological assemblage within the biotope is considered to be 'High' and resilience was assessed as 'High', so that this biotope is judged to be 'Not sensitive'. Limpets may be sensitive to even low levels of deposition (see siltation pressure) so that impacts from this pressure will depend on the duration of input and any deposits. | HighHelp | HighHelp | Not sensitiveHelp |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAll marine habitats and benthic species are considered to have a resistance of ‘None’ to this pressure and to be unable to recover from a permanent loss of habitat (resilience is ‘Very low’). Sensitivity within the direct spatial footprint of this pressure is, therefore ‘High’. Although no specific evidence is described confidence in this assessment is ‘High’, due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceThis biotope is characterized by the hard rock substratum to which the key characterizing species, barnacles, limpets, Patella vulgata and Lichina pygmaea, and other species such as Mytilus edulis and algal turfs can firmly attach. A change to a sedimentary substratum would significantly alter the character of the biotope. More subtle changes in substratum type can also lead to indirect effects. Surface roughness, for example, is correlated with settlement in barnacles (Coombes et al., 2015). An artificial substratum may therefore not be equivalent to a natural rock reef habitat. However, artificial hard substrata will, generally be settled by barnacles and may provide ‘stepping stones’ less suitable surfaces such as chalk where post-settlement mortalities are high(Herbert & Hawkins, 2006) or sediments. An increase in mobile surfaces can also indirectly decrease suitable habitats. Shanks & Wright (1986) observed that limpet mortalities were much higher at sites where the supply of loose cobbles and pebbles were greater, leading to increased abrasion through wave action 'throwing' rocks onto surfaces. Sensitivity assessment. A change from hard rock to sedimentary substratum would result in the loss of the biotope as all the important characterizing species require hard surfaces for attachment, and because the biotope is classified as a hard rock biotope. Therefore. resistance is assessed as 'None'. Resilience is assessed as 'Very low' as the pressure represents a permanent change and, hence, sensitivity is assessed as 'High'. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidenceNot relevant to biotopes occurring on bedrock. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceThe species characterizing this biotope are epifauna or epiflora occurring on rock and would be sensitive to the removal of the habitat. However, extraction of rock substratum is considered unlikely and this pressure is considered to be ‘Not relevant’ to hard substratum habitats. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceThe barnacles, limpets and lichen that are the key characterizing species typically occur on the rock surfaces where they will be exposed to abrasion. Although limpets and barnacles are protected by hard shells or plates, abrasion may damage and kill individuals or detach these. All removed barnacles would be expected to die as there is no mechanism for these to reattach. Removal of limpets may result in these being displaced to a less favourable habitat and injuries to foot muscles may prevent reattachment. Evidence for the effects of abrasion is provided by a number of experimental studies on trampling (a source of abrasion) and on abrasion by wave thrown rocks and pebbles. No evidence was found for Lichina pygmaea, but as an attached, erect species it is likely to be damaged or removed by surface abrasion. The effects of trampling on barnacles appear to be variable with some studies not detecting significant differences between trampled and controlled areas (Tyler-Walters & Arnold, 2008). However, this variability may be related to differences in trampling intensities and abundance of populations studied. The worst case incidence was reported by Brosnan & Crumrine (1994) who reported that a trampling pressure of 250 steps in a 20x20 cm plot one day a month for a period of a year significantly reduced barnacle cover at two study sites. Barnacle cover reduced from 66% to 7% cover in 4 months at one site and from 21% to 5% within 6 months at the second site. Overall barnacles were crushed and removed by trampling. Barnacle cover remained low until recruitment the following spring. Long et al. (2011) also found that heavy trampling (70 humans km-1 shoreline h-1) led to reductions in barnacle cover. Single step experiments provide a clearer, quantitative indication of sensitivity to direct abrasion. Povey & Keough (1991) in experiments on shores in Mornington Peninsula, Victora, Australia, found that in single step experiments 10 out of 67 barnacles (Chthamlus antennatus about 3mm long) were crushed. However, on the same shore, the authors found that limpets may be relatively more resistant to abrasion from trampling. Following step and kicking experiments, few individuals of the limpet Cellana trasomerica, (similar size to Patella vulgata) suffered damage or relocated (Povey & Keough, 1991). One kicked limpet (out of 80) was broken and 2 (out of 80) limpets that were stepped on could not be relocated the following day (Povey & Keough, 1991). Trampling may lead to indirect effects on limpet populations, Bertocci et al. (2011) found that the effects of trampling on Patella sp. increased the temporal and spatial variability of in abundance. The experimental plots were sited on a wave-sheltered shore dominated by Ascophyllum nodosum. On these types of shore, trampling in small patches, that removes macroalgae and turfs, will indirectly enhance habitat suitability for limpets by creating patches of exposed rock for grazing. Shanks & Wright (1986), found that even small pebbles (<6 cm) that were thrown by wave action in Southern California shores could create patches in Chthamalus fissus aggregations and could smash owl limpets (Lottia gigantea). Average, estimated survivorship of limpets at a wave exposed site, with many loose cobbles and pebbles allowing greater levels of abrasion was 40% lower than at a sheltered site. Severe storms were observed to lead to the almost total destruction of local populations of limpets through abrasion by large rocks and boulders. Sensitivity assessment. The impact of surface abrasion will depend on the footprint, duration and magnitude of the pressure. Surface abrasion may directly crush and remove the key characterizing lichen, barnacles and Patella vulgata. Resistance is, therefore, assessed as ‘Low’ for characterizing species. Recovery of the biological assemblage (following habitat restoration) is considered to be 'Medium' (2-10 years) for the variant biotope LR.HLR.MusB.Cht.Cht and 'Low' to 'Very low' for the biotope LR.HLR.MusB.Cht.Lpyg, based on the very slow recovery of Lichina pygmaea. Sensitivity is, therefore, assessed as 'Medium' and 'High', respectively and the assessment for LR.HLR.MusB.Cht.Lpyg is presented. | LowHelp | Very LowHelp | HighHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidenceThe species characterizing this biotope group are epifauna or epiflora occurring on rock, which is resistant to subsurface penetration. Therefore, ‘penetration’ is 'Not relevant'. The assessment for abrasion at the surface only is, therefore, considered to equally represent sensitivity to this pressure’. Please refer to ‘abrasion’ above. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceIn general, increased suspended particles may enhance food supply (where these are organic in origin) or decrease feeding efficiency (where the particles are inorganic and require greater filtration efforts). Very high levels of silt may clog respiratory and feeding organs of the suspension feeding barnacles. In addition, increased turbidity will decrease light penetration reducing photosynthesis by macroalgae within this biotope. Increased levels of particles may increase scour and deposition in the biotope depending on local hydrodynamic conditions, although changes in substratum are assessed through the physical change (to another seabed type) pressure. Gyory et al. (2013) found that increased turbidity triggered the release of larvae by Semibalanus balanoides, a response which may allow the larval release to be timed with high levels of phytoplankton and at times where predation on larvae may be lowered due to the concentration of particles. Storm events that stir up sediments are also associated with the larval release (Gyory & Pineda, 2011). A significant decrease in suspended organic particles may reduce food input resulting in reduced growth and fecundity of the suspension feeding barnacles. However, local primary productivity (phytoplankton and diatom films) may be enhanced where suspended sediments decrease, increasing food supply to both characterizing species. Decreased suspended sediment may increase macroalgal competition, enhancing diversity, but is considered unlikely to significantly change the character of the biotope as colonisation by larger brown macroalgae is likely to be limited by wave action in this biotope. Sensitivity assessment. The benchmark for this pressure refers to a change in turbidity of one rank on the Water Framework Directive (WFD) scale. Where changes in suspended sediment supply were linked to decreased wave action and water flow to enhance settlement, limpets would be sensitive to deposition (see siltation pressures). At the pressure benchmark, however, resistance is assessed as 'High' and resilience as 'High' and the biotope is considered to be 'Not sensitive'.
| HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceMore direct evidence to assess this pressure was found for the characterizing species Patella vulgata, than the barnacles. That barnacles are likely to be sensitive to siltation is shown by the lower limits of Semibalanus balanoides (as Balanus balanoides) that appear to be set by levels of sand inundation on sand-affected rocky shores in New Hamshire (Daly & Mathieson, 1977). Field observations and laboratory experiments have highlighted the sensitivity of limpets to sediment deposition (see also the ‘heavy’ siltation pressure for further information). Airoldi & Hawkins (2007) tested the effects of different grain sizes and deposit thickness in laboratory experiments using Patella vulgata. Sediments were added as a ‘fine’ rain to achieve deposit thicknesses of approximately 1 mm, 2 mm, and 4 mm in controlled experiments and grazing and mortality observed over 8-12 days. Limpets were more sensitive to sediments with a higher fraction of fines (67% silt) than coarse (58% sand). Coarse sediments of thicknesses approximately 1, 2 and 4 mm decreased grazing activity by 35, 45 and 50% respectively. At 1 and 2 mm thicknesses, fine sediments decreased grazing by 40 and 77%. The addition of approximately 4 mm of fine sediment completely inhibited grazing. Limpets tried to escape the sediment but lost attachment and died after a few days (Airoldi & Hawkins, 2007). Observations on exposed and sheltered shores with patches of sediment around Plymouth in the south west of England found that Patella vulgata abundances were higher where deposits were absent. The limpets were locally absent in plots with 50-65% sediment cover (Airoldi & Hawkins, 2007). Littler et al. (1983) found that another limpet species, Lottia gigantea on southern Californian shores was restricted to refuges from sand burial on shores subject to periodic inundation by sands. Sensitivity assessment. The barnacles that characterize this biotope are found permanently attached to hard substrata and are suspension feeders. Therefore, they have no ability to escape from silty sediments which would bury individuals and prevent feeding and respiration. However, no direct evidence for sensitivity to siltation was found. Resistance is assessed as ‘Medium’ as wave action on rocky shores is likely to rapidly mobilise and remove deposits alleviating the effect of smothering. Resilience is assessed as ‘High’ and sensitivity is therefore considered to be ‘Low’. Even small deposits of sediments are likely to result in local removal of limpets. The level of impact will depend on the magnitude and duration of impact. It should be noted that the level of exposure may be reduced by wave action or water flows so that site-specific vulnerability will be lower where sediments do not accumulate. Resistance to siltation is assessed as ‘Low’ for Patella vulgata based primarily on observations and experiments of Airoldi & Hawkins, (2007), who demonstrated negative effects at deposit thicknesses far lower than the pressure benchmark. Small patches subject to a single impact may recover rapidly via adult migration. However, based on the prolonged recovery times experienced in more wide-ranging impacts, resilience is assessed as ‘Medium’ (2-10 years) and sensitivity is therefore assessed as ‘Medium’. This more precautionary assessment is presented for the biotope, rather than the lower sensitivity of Semibalanus balanoides. Repeated deposition events, coupled with changes in water flow and wave action may lead to the establishment of turf-forming algae that trap sediments, this would significantly alter the character of the biotope. | LowHelp | MediumHelp | MediumHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceMore direct evidence to assess this pressure was found for the characterizing species Patella vulgata, than the barnacles. That barnacles are likely to be sensitive to siltation is shown by the lower limits of Semibalanus balanoides (as Balanus balanoides) that appear to be set by levels of sand inundation on sand-affected rocky shores in New Hamshire (Daly & Mathieson, 1977). Field observations and laboratory experiments have highlighted the sensitivity of limpets to sediment deposition (see also the ‘heavy’ siltation pressure for further information). Airoldi & Hawkins (2007) tested the effects of different grain sizes and deposit thickness in laboratory experiments using Patella vulgata. Sediments were added as a ‘fine’ rain to achieve deposit thicknesses of approximately 1 mm, 2 mm, and 4 mm in controlled experiments and grazing and mortality observed over 8-12 days. Limpets were more sensitive to sediments with a higher faction of fines (67% silt) than coarse (58% sand). Coarse sediments of thicknesses approximately 1, 2 and 4 mm decreased grazing activity by 35, 45 and 50 % respectively. At 1 and 2 mm thicknesses, fine sediments decreased grazing by 40 and 77%. The addition of approximately 4 mm of fine sediment completely inhibited grazing. Limpets tried to escape the sediment but lost attachment and died after a few days (Airoldi & Hawkins, 2007). Observations on exposed and sheltered shores with patches of sediment around Plymouth in the south-west of England found that Patella vulgata abundances were higher where deposits were absent. The limpets were locally absent in plots with 50-65% sediment cover (Airoldi & Hawkins, 2007). Littler et al. (1983) found that another limpet species, Lottia gigantea on southern Californian shores was restricted to refuges from sand burial on shores subject to periodic inundation by sands. Sensitivity assessment. Sensitivity to this pressure will be mediated by site-specific hydrodynamic conditions and the footprint of the impact. Where a large area is covered sediments may be shifted by wave and tides rather than removed. The barnacles are permanently attached to hard substrates and therefore have no ability to escape from silty sediments which will prevent feeding and respiration. No evidence was found to assess this pressure and resistance was assessed as 'Low' as substantial mortality is considered likely to occur from scour effects and smothering. Resilience is assessed as ‘Medium’ and sensitivity is therefore considered to be ‘Medium’. However, mortality will depend on the duration of smothering, where wave action rapidly mobilises and removes fine sediments, survival may be much greater. Even small deposits of sediments are likely to result in local removal of limpets. Resistance to siltation at the benchmark level is assessed as ‘None’ for Patella vulgata based primarily on the observations and experiments of Airoldi & Hawkins, (2007), who demonstrated negative effects at deposit thicknesses far lower than the pressure benchmark. Small patches subject to a single impact may recover rapidly via adult migration. However, based on the prolonged recovery times experienced in more wide-ranging impacts, resilience is assessed as ‘Medium’ (2-10 years) and sensitivity is therefore assessed as ‘Medium’. Repeated deposition events, coupled with changes in water flow and wave action may lead to the establishment of turf-forming algae that trap sediments, this would significantly alter the character of the biotope. | NoneHelp | MediumHelp | MediumHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail EvidenceThompson et al. (2004) demonstrated that Semibalanus balanoides kept in aquaria, ingested microplastics within a few days. However, the effects of the microplastics on the health of exposed individuals have not been identified. There is currently no evidence to assess the level of impact. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail EvidenceNo evidence. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceNot relevant. Wave action on exposed shores is likely to generate high levels of underwater noise. Other sources are not considered likely to result in effects on the biotope. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidenceSemibalanus balanoides sheltered from the sun grew bigger than unshaded individuals (Hatton, 1938; cited in Wethey, 1984), although the effect may be due to indirect cooling effects rather than shading. Barnacles are also frequently found under algal canopies suggesting that they are tolerant of shading. Light levels have also been demonstrated to influence a number of phases of the reproductive cycle in Semibalanus balanoides. In general, light inhibits aspects of the breeding cycle. Penis development is inhibited by light (Barnes & Stone, 1972) while Tighe-Ford (1967) showed that constant light inhibited gonad maturation and fertilization. Davenport & Crisp (unpublished data from Menai Bridge, Wales, cited from Davenport et al., 2005) found that experimental exposure to either constant darkness or 6-hour light: 18-hour dark photoperiods induced autumn breeding in Semibalanus. They also confirmed that very low continuous light intensities (little more than starlight) inhibited breeding. Latitudinal variations in the timing of the onset of reproductive phases (egg mass hardening) have been linked to the length of darkness (night) experienced by individuals rather than temperature (Davenport et al., 2005). Changes in light levels associated with climate change (increased cloud cover) were considered to have the potential to alter the timing of reproduction (Davenport et al., 2005) and to shift the range limits of this species southward. However, it is not clear how these findings may reflect changes in light levels from artificial sources, and whether observable changes would occur at the population level as a result. There is, therefore, 'insufficient evidence' on which to base an assessment. | Insufficient evidence (IEv)Help | Not relevant (NR)Help | Help |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceNo direct evidence was found to assess this pressure. As the larvae of Patella vulgata and Chthamalus spp. are planktonic and are transported by water movements, barriers that reduce the degree of tidal excursion may alter larval supply to suitable habitats from source populations. However, the presence of barriers may enhance local population supply by preventing the loss of larvae from enclosed habitats. As both species are widely distributed and have larvae capable of long distance transport, resistance to this pressure is assessed as 'High' and resilience as 'High' by default. This biotope is therefore considered to be 'Not sensitive'.
| HighHelp | HighHelp | Not sensitiveHelp |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceNot relevant’ to seabed habitats. NB. Collision by grounding vessels is addressed under ‘surface abrasion. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceNot relevant. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidenceThe characterizing species, Chthamalus spp. and Patella vulgata and other common rocky shores species within the biotope, with the exception of Mytilus edulis which occurs in low densities, are not subject to translocation or cultivation. Commercial cultivation of Mytilus edulis involves the collection of juvenile mussel ‘seed’ or spat (newly settled juveniles ca 1-2cm in length) from wild populations, with subsequent transportation around the UK for re-laying in suitable habitats. As the seed is harvested from wild populations from various locations the gene pool will not necessarily be decreased by translocations. Movement of mussel seed has the potential to transport pathogens and non-native species (see sensitivity assessments for Mytilus edulis bed biotopes). A review by Svåsand et al. (2007) concluded that there was a lack of evidence distinguishing between different Mytilus edulis populations to accurately assess the impacts of hybridization with the congener Mytilus galloprovincialis and in particular how the gene flow may be affected by aquaculture. Therefore, it cannot be confirmed whether farming will have an impact on the genetics of this species beyond a potential for increased hybridisation. Sensitivity assessment. No direct evidence was found regarding the potential for negative impacts of translocated mussel seed on wild Mytilus edulis populations. While it is possible that translocation of mussel seed could lead to gene flow between cultivated beds and local wild populations, there is currently no evidence to assess the impact (Svåsand et al., 2007). | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidenceThe characterizing barnacles and limpets Patella vulgata are considered subject to persistent, low levels of infection by pathogens and parasites. Patella vulgata has been reported to be infected by the protozoan Urceolaria patellae (Brouardel, 1948) at sites sheltered from extreme wave action in Orkney. Baxter (1984) found shells to be infested with two boring organisms, the polychaete Polydora ciliata and a siliceous sponge Cliona celata. No evidence was found for microbial pathogen infection in Lichina pygmaea. Sensitivity assessment. Based on the characterizing species and the lack of evidence for widespread, high-level mortality due to microbial pathogens the biotope is considered to have 'High' resistance to this pressure and therefore 'High' resilience (by default), the biotope is therefore considered to be 'Not sensitive'. | MediumHelp | HighHelp | LowHelp |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceThe species Mytilus edulis is too small and patchy in this biotope to be targeted for commercial harvesting. However, some, unregulated recreational hand-gathering of this species and the limpet Patella vulgata, may occur. Gathering of Mytilus edulis is not considered to affect the biotope as this species is present in low densities, as small individuals in cracks and crevices and is, therefore, not a key characterizing or structuring species. Patella vulgata, however, is a key characterizing and structuring species within the variant biotope LR.HLR.MusB.Cht.Cht. Patella vulgata grazing can control the character of the shore by grazing algae and newly settled barnacle larvae. Even a small, localised temporary absence of limpets (Southward, 1956; Southward, 1964; Hawkins, 1981; Hawkins et al., 1983) can alter the biological assemblage. Significant limpet kills resulting from the widespread use of dispersants after the Torrey Canyon oil spill dramatically altered rocky shore communities allowing dense growths of ephemeral green seaweeds followed by equally dense growth of fucoids (Southward & Southward, 1978; Hawkins & Southward, 1992). Sensitivity assessment. Patella vulgata is considered a key structuring species within the barnacle and limpet dominated biotope, LR.HLR.MusB.Cht.Cht, as its grazing (coupled with wave action), maintains the bare rock surfaces preventing a dense algal turf from developing and allowing colonization by Chthamalus species. The resistance of Patella vulgata to removal is 'Low' as this species is relatively large and is immobile and, therefore, easily found and removed. Recovery (of the species and biotope) is assessed as 'Low' (10-20) years as an alternate stable state may develop (see resilience section) so that sensitivity is assessed as 'High'. It should be noted that the assessment refers to a single event, sensitivity to persistent, on-going collection would be higher. The variant biotope, LR.HLR.MusB.Cht.Lpyg generally occurs at higher shore levels where Patella vulgata are less abundant and where grazing is probably of lower importance compared to emergence and wave action in controlling macroalgae. However the assessment for LR.HLR.MusB.Cht.Cht is considered equally applicable, as fucoid growth and competition can occur at the height of the biotope on some shores (Boney, 1961). | LowHelp | LowHelp | HighHelp |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidenceThe characterizing species Mytilus edulis is too small and patchy in this biotope to be targeted for commercial harvesting. However, some hand-gathering of this species may occur. Incidental removal of the characterizing species, lichens, limpets and barnacles would alter the character of the biotope. The ecological services such as filtration and primary and secondary production provided by these species would also be lost. Sensitivity assessment. Removal of a large percentage of the characterizing species would alter the character of the biotope so that it was bare rock. Resistance is, therefore, assessed as ‘Low’. Resilience is assessed as ‘Medium’ for the variant biotope LR.HLR.MusB.Cht.Cht and 'Low' to 'Very Low' for the variant biotope LR.HLR.MusB.Cht.Lpyg and sensitivity is, therefore, assessed as 'Medium' and 'High', respectively. The higher sensitivity is recorded. | LowHelp | LowHelp | HighHelp |
Introduction or spread of invasive non-indigenous species (INIS) Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Other INIS [Show more]Other INISEvidenceThe Pacific oyster Magallana (syn. Crassostrea) gigas is native to warm temperate regions from the northwest Pacific to Japan and northeast Asia, including Cape Mariya (Russia) to Hong Kong (China) (Carrasco & Baron, 2010; GBNNSS, 2011, 2012). It is a fast-growing and tolerant species that has become a successful invader in the coastal waters of all continents, aside from Antarctica (Wrange et al., 2010; Carrasco & Baron, 2010; Padilla, 2010). Magallana gigas is recognised as a beneficial and important species in aquaculture worldwide (Padilla, 2010). It was initially introduced for aquaculture in Europe and the UK in the 1960s due to a decline in the Portuguese oyster (Crassostrea angulata) and the European flat oyster (Ostrea edulis) (Spencer et al., 1994; GBNNSS, 2011, 2012; Humphreys et al., 2014 cited in Alves et al., 2021; Hansen et al., 2023). Since introduction, the species has invaded and established self-sustaining natural populations throughout Europe from the North Sea, Wadden Sea and Scandinavian coastlines to the Atlantic coastlines of Spain and Portugal, as well as the Mediterranean and Adriatic Sea (Wrange et al., 2010; GBNNSS, 2011, 2012; Ezgeta-Balic et al., 2019; Spagnolo et al., 2019; Bergstrom et al., 2021; Hansen et al., 2023). In the UK, the species predominantly occurs around the southern and western coastlines (OBIS, 2024; NBN, 2024). Shipping activity has also been associated with the introduction of Magallana gigas in the northeastern Adriatic Sea, where it was not introduced for aquaculture (Ezgeta-Balic et al., 2019). It was also suggested that some Magallana gigas populations were established in southwest England from France possibly via fouling on ships (GBNNSS, 2011, 2012; Padilla, 2010; Ezgeta-Balic et al., 2019). Magallana gigas has a high fecundity, a long-lived pelagic larval phase (2 to 4 weeks) and can produce up to 200 million eggs during spawning (Herbert et al., 2012, 2016; Alves et al., 2021; Wood et al., 2021; Hansen et al., 2023). Hence, as a broadcast spawner, it has a high dispersal potential of more than 1000 km (Padilla, 2010; Wood et al., 2021). Larval mortality can be as large as 99%, as larvae are sensitive to environmental conditions (Alves et al., 2021). However, adults are long-lived so populations can survive with infrequent recruitment (Padilla, 2010). Larval dispersal and mass spawning events have facilitated the settlement and establishment of Pacific oysters, as seen in the Oosterschelde estuary, Netherlands (Hansen et al., 2023). It has been suggested that the spread of the Pacific oyster in Scandinavia is due to northward larval drift on tidal and wind-driven currents (Hansen et al., 2023). Wood et al. (2021) suggested that larval dispersal of the Pacific oyster from populations within and outside the UK was possible via unaided (passive) transport by currents, but that aquaculture and offshore structures (e.g. windfarms) increased the risk of the invasive species spreading and the geographical extent of spread. Magallana gigas is an ecosystem engineer and can dramatically change habitat structure when it invades. Once successfully settled, groups of Pacific oysters may form dense aggregations, potentially forming a reef, which in some regions can reach densities of 700 individuals/m2 (Herbert et al., 2012, 2016). Once, the density of live or dead Pacific oysters reaches or exceeds 200 ind./m2, little of the underlying substratum remains visible (Herbert et al., 2016). These reefs can stabilize the sediment surface locally (Troost, 2010). When such reefs are formed or, particularly when the species colonizes soft sediments such as mud or sand, it can change and affect local communities, by creating hard substrata for mobile species, which might not otherwise be present before the invasion (Padilla, 2010). However, Hansen et al. (2023) suggested that where the Pacific oyster occurs sporadically, no immediate ecosystem risk is observed. Magallana gigas also colonizes littoral intertidal biogenic reefs formed by the blue mussel Mytilus edulis or honeycomb worm Sabellaria alveolata (GBNNSS, 2011, 2012; Kochmann, 2012; Kochmann et al., 2013; Herbert et al., 2016; Tillin et al., 2020). Evidence suggests the Pacific oyster can out-compete Mytilus edulis, particularly for food and space, as the faster growth rates of the oyster make it more competitive when food or space is limiting (Nehls et al., 2006; Padilla, 2010; Tillin et al., 2020; Joyce et al., 2021). The invasion of Magallana gigas may alter the structure and function of these intertidal reefs but can create a multi-layered structure of a mixture of oysters and blue mussels that is more resilient and accumulates a higher biodiversity of flora and fauna and supports the densities of other native species such as Littorina littorea (Reise et al., 2017; Andriana et al., 2020; Cornelius & Buschbaum, 2020; Hansen et al., 2023). Magallana gigas requires hard substrata for successful settlement and establishment, including littoral rock, bedrock, chalk, bare boulders, cobbles and pebbles and shells (Kochmann, 2012; Kochmann et al., 2013; Mckinstry & Jensen, 2013; Herbert et al., 2016; Tillin et al., 2020) because its larvae require hard substrata for successful settlement and development (Mckinstry & Jensen, 2013; Tillin et al., 2020). Invasive populations of Magallana gigas have been found wave-exposed rocky shores to wave-sheltered soft sediment environments and it has been described as a habitat generalist (Troost, 2010; Kochmann, 2012; Kochmann et al., 2013). For example, in Scotland, wild Magallana gigas are mainly located in the lower intertidal on bedrock, bedrock encrusted with barnacles, within bedrock crevices, and large and small boulders (Cook et al., 2014). They are unlikely to occur under boulders as they require access to the water column (Tillin et al., 2020). Patches of Pacific oyster reefs have been recorded on littoral rock in Kent, southern England and on littoral sediments in southern England, the North Sea and the English Channel (Herbert et al., 2012, 2016; Morgan et al., 2021). On littoral rock in Brittany, the Pacific oyster colonizes all intertidal levels from Mean High Water to Mean Low Water on sheltered (low energy), moderately exposed (moderate energy) and high energy rock shores (Herbert et al., 2012). However, in the northwest Pacific, Magallana gigas is commonly found on sheltered low energy littoral rock and has less than 10% cover on exposed high energy littoral rock shores (Herbert et al., 2012, 2016). Magallana gigas has not been found at extreme low water levels or subtidally beneath rocky habitats, as it has been in soft sediment areas (Herbert et al., 2012). It has been suggested that recruitment is enhanced and abundances are higher in wave-sheltered conditions (Robinson et al., 2005; Ruesink, 2007 cited in Teschke et al., 2020; Tillin et al., 2020). Teschke et al. (2020) found the abundance of Magallana gigas was significantly higher at wave-protected sites within the artificial harbours of Helgoland, North Sea, compared to wave exposed sites outside the harbours. In addition, better growth and higher survival rates were observed at wave-protected sites, whereas mortality rates increased at wave exposed sites, due to the wave exposure causing dislodgement or detachment from the settlement substratum (Teschke et al., 2020; Tillin et al., 2020). Similarly, Bergstrom et al. (2021) noted that the occurrence of high densities of both Ostrea edulis and Magallana gigas decreased with increasing wave exposure. In the Bay of Brest, Pacific oyster reefs on rock had a greater diversity, species richness and biomass than the surrounding bare rock habitats (Lejart & Hily, 2011). There was an increase in macrograzers, suspension feeders, carnivores, deposit and detritus feeders in the present on oyster reefs on rock compared with the surrounding bare rock (Lejart & Hily, 2011). Their results showed that 15% of species present in the oyster reefs on rock were characteristic of mud habitats (Lejart & Hily, 2011). Lejart & Hily (2011) found the surface available for epibenthic species in the Bay of Brest, increased 4-fold when oysters were present on rock, for every 1 m2 of colonized substrata the oyster reef added 3.97 m2 of surface area on rock. An increase in available settlement substrata, which is free of epibiota, could be why oyster reefs cause an increase in the macrofaunal abundance. Zwerschke et al. (2018) found at intertidal rocky sites and sites with gravel around the UK, Ireland and northern France, densities of Pacific oysters more than 10 m2 had a different macrofaunal assemblage structure than sites with low density or no Magallana gigas. Their results showed a greater abundance of species such as barnacles in mud, rock and gravel sites when Pacific oysters were superabundant (oyster density more than 99 /m2). However, a decrease in the abundance of kelp, Fucus vesiculosus and periwinkle Littorina sp. was observed on the rocky shore sites colonized by the oysters (Zwerschke et al., 2018). In addition, the settlement of Magallana gigas in the barnacle zone of exposed rocky shores in the Strait of Georgia, Canada provided a greater surface area for settlement while neighbouring species at the rocky sites facilitated the survival of the Pacific oyster, by reducing predation and physical stress (Ruesink et al., 2005; Herbert et al., 2012). Similarly, in rocky habitats, in Argentina, four epifaunal species (crabs Cyrtograpsus angulatus, Chasmagnathus granulatus, isopod Melita palmata and snail Helebia australis) showered higher densities and abundance within Magallana gigas beds than outside these areas (Escapa et al., 2004; Herbert et al., 2012). The Australasian barnacle Austrominius (previously Elminius) modestus was introduced to British waters on ships during the Second World War. However, its overall effect on the dynamics of rocky shores has been small as Austrominius modestus has simply replaced some individuals of a group of co-occurring barnacles (Raffaelli & Hawkins, 1999). Although present monitoring indicates it has not outnumbered native barnacles in the Isle of Cumbrae (Gallagher et al., 2015), it may dominate in estuaries where it is more tolerant of lower salinities than Semibalanus balanoides (Gomes-Filho, et al., 2010). The degree of wave exposure experienced by this biotope will limit colonization by Austrominius modestus, which tends to be present in more sheltered biotopes. The wave exposure and shore height are also considered to be unsuitable for many invasive, non-native species. The non-native crab Hemigrapsus sanguineus has recently been recorded in the UK (Sweet & Sewell, 2014) and has the potential to be a significant predator of intertidal invertebrates. Significant reductions in common shore crab abundance and mussel density have been reported where the Asian shore crab has achieved high densities in mainland Europe (Sweet & Sewell, 2014). However, Brousseau & Goldberg (2007) found that even at high crab densities the effects of predation on the density of Semibalanus balanoides were limited as continued recruitment offset predation. These results may be applicable to Chthamalus sp. and the height on the shore may limit crab feeding times. Sensitivity assessment. The Pacific oyster Magallana gigas can colonize all intertidal levels on littoral rock (Herbert et al., 2012) and result in a change in the community depending on density (Ruesink et al., 2005; Lejart & Hily, 2011; Herbert et al., 2012; Zwerschke et al., 2018). The biotope may be altered or replaced. However, Magallana gigas populations may be limited to low densities due to very wave exposed to moderately wave exposed conditions (Teschke et al., 2020). On steep or vertical examples of the biotope, densities of Magallana gigas may also be limited as these conditions are less suitable for colonization, suggesting a resistance of ‘Medium’. However, other flat and shallow-sloped examples of the biotope are more suitable for colonization. Therefore, a precautionary resistance of ‘Low’ is suggested for intertidal rock biotopes. Resilience is likely to be ‘Very low’ as Magallana gigas would need to be physically removed for recovery to occur. Therefore, sensitivity is assessed as ‘High’ for intertidal rock biotopes. | LowHelp | Very LowHelp | HighHelp |
Bibliography
Airoldi, L. & Hawkins, S.J., 2007. Negative effects of sediment deposition on grazing activity and survival of the limpet Patella vulgata. Marine Ecology Progress Series, 332, 235-240. DOI https://doi.org/10.3354/meps332235
Alfaro, A.C., 2006. Byssal attachment of juvenile mussels, Perna canaliculus, affected by water motion and air bubbles. Aquaculture, 255, 357-61
Almada-Villela, P.C., Davenport, J. & Gruffydd, L.L.D., 1982. The effects of temperature on the shell growth of young Mytilus edulis L. Journal of Experimental Marine Biology and Ecology, 59, 275-288.
Alves, M. T., Taylor, N. G. H. & Tidbury, H. J., 2021. Understanding drivers of wild oyster population persistence. Sci Rep, 11 (1), 7837. DOI https://doi.org/10.1038/s41598-021-87418-1
Andriana, R., van der Ouderaa, I. & Eriksson, B. K., 2020. A Pacific oyster invasion transforms shellfish reef structure by changing the development of associated seaweeds. Estuarine Coastal and Shelf Science, 235. DOI https://doi.org/10.1016/j.ecss.2019.106564
Arnold, D.C., 1957. The response of the limpet, Patella vulgata L., to waters of different salinities. Journal of the Marine Biological Association of the United Kingdom, 36, 121-128.
Barnes, H., 1956. Balanus balanoides (L.) in the Firth of Clyde: the development and annual variation in the larval population and the causative factors. Journal of Animal Ecology, 25, 72-84.
Barnes, H. & Barnes, M., 1974. The responses during development of the embryos of some common cirripedes to wide changes in salinity. Journal of Experimental Marine Biology and Ecology, 15 (2), 197-202.
Barnes, H. & Barnes, M., 1965. Egg size, nauplius size, and their variation with local, geographical and specific factors in some common cirripedes. Journal of Animal Ecology, 34, 391-402.
Barnes, H. & Stone, R., 1972. Suppression of penis development in Balanus balanoides (L.). Journal of Experimental Marine Biology and Ecology, 9 (3), 303-309.
Barnes, H., 1953. The effect of lowered salinity on some barnacle nauplii. Journal of Animal Ecology, 22, 328-330.
Barnes, H., 1957. Processes of restoration and synchronization in marine ecology. The spring diatom increase and the 'spawning' of the common barnacle Balanus balanoides (L.). Année Biologique. Paris, 33, 68-85.
Barnes, H., Finlayson, D.M. & Piatigorsky, J., 1963. The effect of desiccation and anaerobic conditions on the behaviour, survival and general metabolism of three common cirripedes. Journal of Animal Ecology, 32, 233-252.
Barnes, M., 1989. Egg production in Cirripedia. Oceanography and Marine Biology: an Annual Review, 27, 91-166.
Barnes, M., 2000. The use of intertidal barnacle shells. Oceanography and Marine Biology: an Annual Review, 38, 157-187.
Baxter, J.M., 1984. The incidence of Polydora ciliata and Cliona celata boring the shell of Patella vulgata in Orkney. Journal of the Marine Biological Association of the United Kingdom, 64, 728-729.
Bayne, B.L., 1976a. The biology of mussel larvae. In Marine mussels: their ecology and physiology (ed. B.L. Bayne), pp. 81-120. Cambridge: Cambridge University Press. [International Biological Programme 10.]
Bennell, S.J., 1981. Some observations on the littoral barnacle populations of North Wales. Marine Environmental Research, 5, 227-240.
Bergmann, M., Wieczorek, S.K., Moore, P.G., 2002. Utilisation of invertebrates discarded from the Nephrops fishery by variously selective benthic scavengers in the west of Scotland. Marine Ecology Progress Series, 233,185-98
Bergström, P., Thorngren, L., Strand, Å & Lindegarth, M., 2021. Identifying high-density areas of oysters using species distribution modeling: Lessons for conservation of the native Ostrea edulis and management of the invasive Magallana (Crassostrea) gigas in Sweden. Ecology and Evolution, 11 (10), 5522-5532. DOI https://doi.org/10.1002/ece3.7451
Berthe, F.C.J., Le Roux, F., Adlard, R.D. & Figueras, A., 2004. Marteiliosis in molluscs: a review. Aquatic Living Resources, 17 (4), 433-448.
Bertness, M.D., 1984. Habitat and community modification by an introduced herbivorous snail. Ecology, 65, 370-381.
Bertness, M.D., Gaines, S. D., Stephens, E. G., & Yund, P. O. , 1992. Components of recruitment in populations of the acorn barnacle Semibalanus balanoides (Linnaeus). Journal of Experimental Marine Biology and Ecology, 156 (2), 199-215.
Bertness, M.D., Gaines, S.D., Bermudez, D. & Sanford, E., 1991. Extreme spatial variation in the growth and reproductive output of the acorn barnacle Semibalanus balanoides. Marine Ecology Progress Series, 75, 91-100.
Bertocci, I., Araujo, R., Vaselli, S. & Sousa-Pinto, I., 2011. Marginal populations under pressure: spatial and temporal heterogeneity of Ascophyllum nodosum and associated assemblages affected by human trampling in Portugal. Marine Ecology Progress Series, 439, 73-82.
Blackmore, D.T., 1969. Growth, reproduction and zonation of Patella vulgata. Journal of Experimental Marine Biology and Ecology, 3, 200-213.
Boney, A., 1961. A note on the intertidal lichen Lichina pygmaea AG. Journal of the Marine Biological Association of the United Kingdom, 41 (01), 123-126.
Boney, A.D., 1979. Long-term observations on the intertidal lichen Lichina pygmaea Ag. Journal of the Marine Biological Association of the UK, 59(3), 801-802.
Bonner, T. M., Pyatt, F. B. & Storey, D. M., 1993. Studies on the motility of the limpet Patella vulgata in acidified sea-water. International Journal of Environmental Studies, 43, 313-320.
Bousfield, E.L., 1973. Shallow-water gammaridean Amphipoda of New England. London: Cornell University Press.
Bower S.M., 2010. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish [online]. Ontario, Fisheries and Oceans, Canada. Available from: http://dev-public.rhq.pac.dfo-mpo.gc.ca/science/species-especes/shellfish-coquillages/diseases-maladies/index-eng.htm [Accessed: 14/02/2014]
Bower, S.M. & McGladdery, S.E., 1996. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish. SeaLane Diseases of Shellfish. [on-line]. http://www-sci.pac.dfo-mpo.gc.ca/sealane/aquac/pages/toc.htm, 2000-11-27
Bower, S.M., 1992. Diseases and parasites of mussels. In The mussel Mytilus: ecology, physiology, genetics and culture (ed. E.M. Gosling), pp. 543-563. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25.]
Bowman, R.S., 1985. The biology of the limpet Patella vulgata L. in the British Isles: spawning time as a factor determining recruitment sucess. In The Ecology of Rocky Coasts: essays presented to J.R. Lewis, D.Sc., (ed. P.G. Moore & R. Seed), Hodder and Stoughton, London, pages 178-193.
Bowman, R.S. and Lewis, J.R., 1986. Geographical variation in the breeding cycles and recruitment of Patella spp. Hydrobiologia, 142, 41-56.
Bowman, R.S. & Lewis, J.R., 1977. Annual fluctuations in the recruitment of Patella vulgata L. Journal of the Marine Biological Association of the United Kingdom, 57, 793-815.
Brawley, S.H., 1992b. Mesoherbivores. In Plant-animal interactions in the marine benthos (ed. D.M John, S.J. Hawkins & J.H. Price), pp. 235-263. Oxford: Clarendon Press. [Systematics Association Special Volume, no. 46.]
Brosnan, D.M., 1993. The effect of human trampling on biodiversity of rocky shores: monitoring and management strategies. Recent Advances in Marine Science and Technology, 1992, 333-341.
Brosnan, D.M. & Crumrine, L.L., 1994. Effects of human trampling on marine rocky shore communities. Journal of Experimental Marine Biology and Ecology, 177, 79-97.
Brouardel, J., 1948. Etude du mode d'infestation des Patelles par Urceolaria patellae (Cuenot): influence de l'espece de Patelle. Bulletin du Laboratoire maritime de Dinard, 30, 1-6.
Brousseau, D.J. & Goldberg, R., 2007. Effect of predation by the invasive crab Hemigrapsus sanguineus on recruiting barnacles Semibalanus balanoides in western Long Island Sound, USA. Marine Ecology Progress Series, 339, 221-228.
Brown, P.J. & Taylor, R.B., 1999. Effects of trampling by humans on animals inhabiting coralline algal turf in the rocky intertidal. Journal of Experimental Marine Biology and Ecology, 235, 45-53.
Browne, M.A., Dissanayake, A., Galloway, T.S., Lowe, D.M. & Thompson, R.C., 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental Science & Technology, 42 (13), 5026-5031.
Bryan, G.W. & Gibbs, P.E., 1983. Heavy metals from the Fal estuary, Cornwall: a study of long-term contamination by mining waste and its effects on estuarine organisms. Plymouth: Marine Biological Association of the United Kingdom. [Occasional Publication, no. 2.]
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Burrows, E.M., 1991. Seaweeds of the British Isles. Volume 2. Chlorophyta. London: British Museum (Natural History).
Burrows, M.T., Hawkins, S.J. & Southward, A.J., 1992. A comparison of reproduction in co-occurring chthamalid barnacles, Chthamalus stellatus (Poli) and Chthamalus montagui Southward. Journal of Experimental Marine Biology and Ecology, 160, 229-249.
Cabral-Oliveira, J., Mendes, S., Maranhão, P. & Pardal, M., 2014. Effects of sewage pollution on the structure of rocky shore macroinvertebrate assemblages. Hydrobiologia, 726 (1), 271-283.
Carrasco, Mauro F. & Barón, Pedro J., 2010. Analysis of the potential geographic range of the Pacific oyster Crassostrea gigas (Thunberg, 1793) based on surface seawater temperature satellite data and climate charts: the coast of South America as a study case. Biological Invasions, 12 (8), 2597-2607. DOI https://doi.org/10.1007/s10530-009-9668-0
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Connell, J.H., 1961b. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology, 42 (4), 710-723.
Connell, J.H., 1961. Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecological Monographs, 31, 61-104.
Connor, D.W., Allen, J.H., Golding, N., Howell, K.L., Lieberknecht, L.M., Northen, K.O. & Reker, J.B., 2004. The Marine Habitat Classification for Britain and Ireland. Version 04.05. ISBN 1 861 07561 8. In JNCC (2015), The Marine Habitat Classification for Britain and Ireland Version 15.03. [2019-07-24]. Joint Nature Conservation Committee, Peterborough. Available from https://mhc.jncc.gov.uk/
Connor, D.W., Brazier, D.P., Hill, T.O., & Northen, K.O., 1997b. Marine biotope classification for Britain and Ireland. Vol. 1. Littoral biotopes. Joint Nature Conservation Committee, Peterborough, JNCC Report no. 229, Version 97.06., Joint Nature Conservation Committee, Peterborough, JNCC Report No. 230, Version 97.06.
Cook, E., Beveridge, C., Lamont, P., O'Higgins, T. & Wilding, T., 2014. Survey of wild Pacific Oyster (Crassostrea gigas) in Scotland. Scottish Aquaculture Research Forum. DOI https://doi.org/10.13140/RG.2.1.1371.7369
Coombes, M.A., La Marca, E.C., Naylor, L.A. & Thompson, R.C., 2015. Getting into the groove: Opportunities to enhance the ecological value of hard coastal infrastructure using fine-scale surface textures. Ecological Engineering, 77, 314-323.
Cornelius, A. & Buschbaum, C., 2020. Introduced marine ecosystem engineers change native biotic habitats but not necessarily associated species interactions. Estuarine Coastal and Shelf Science, 245. DOI https://doi.org/10.1016/j.ecss.2020.106936
Crisp, D., 1961. Territorial behaviour in barnacle settlement. Journal of Experimental Biology, 38 (2), 429-446.
Crisp, D. & Patel, B., 1969. Environmental control of the breeding of three boreo-arctic cirripedes. Marine Biology, 2 (3), 283-295.
Crisp, D.J. & Southward, A.J., 1961. Different types of cirral activity Philosophical Transactions of the Royal Society of London, Series B, 243, 271-308.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crisp, D.J., Southward, A.J. & Southward, E.C., 1981. On the distribution of the intertidal barnacles Chthamalus stellatus, Chthamalus montagui and Euraphia depressa. Journal of the Marine Biological Association of the United Kingdom, 61, 359-380.
Crothers, J.H., 1985. Dog-whelks: an introduction to the biology of Nucella lapillus (L.) Field Studies, 6, 291-360.
Daly, M.A. & Mathieson, A.C., 1977. The effects of sand movement on intertidal seaweeds and selected invertebrates at Bound Rock, New Hampshire, USA. Marine Biology, 43, 45-55.
Dame, R.F.D., 1996. Ecology of Marine Bivalves: an Ecosystem Approach. New York: CRC Press Inc. [Marine Science Series.]
Dare, P.J., 1976. Settlement, growth and production of the mussel, Mytilus edulis L., in Morecambe Bay, England. Fishery Investigations, Ministry of Agriculture, Fisheries and Food, Series II, 28 , 25pp.
Davenport, J. & Davenport, J.L., 2005. Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Marine Ecology Progress Series, 292, 41-50.
Davenport, J., Berggren, M.S., Brattegard, T., Brattenborg, N., Burrows, M., Jenkins, S., McGrath, D., MacNamara, R., Sneli, J.-A. & Walker, G., 2005. Doses of darkness control latitudinal differences in breeding date in the barnacle Semibalanus balanoides. Journal of the Marine Biological Association of the United Kingdom, 85 (01), 59-63.
Davenport, J., Moore, P.G., Magill, S.H. & Fraser, L.A., 1998. Enhanced condition in dogwhelks, Nucella lapillus (L.) living under mussel hummocks. Journal of Experimental Marine Biology and Ecology, 230, 225-234.
Davies, G., Dare, P.J. & Edwards, D.B., 1980. Fenced enclosures for the protection of seed mussels (Mytilus edulis L.) from predation by shore crabs (Carcinus maenas (L.)) in Morecambe Bay, England. Ministry of Agriculture, Fisheries and Food. Fisheries Technical Report, no. 56.
Davies, M.S., 1992. Heavy metals in seawater: effects on limpet pedal mucus production. Water Research, 26, 1691-1693.
Davies, S.P., 1970. Physiological ecology of Patella IV. Environmental and limpet body temperatures. Journal of the Marine Biological Association of the United Kingdom, 50 (04), 1069-1077.
De Vooys, C.G.N., 1987. Elimination of sand in the blue mussel Mytilus edulis. Netherlands Journal of Sea Research, 21, 75-78.
Delany, J., Myers, A., McGrath, D., O'Riordan, R. & Power, A.M., 2003. Role of post-settlement mortality and'supply-side' ecology in setting patterns of intertidal distribution in the chthamalid barnacles Chthamalus montagui and C. stellatus. Marine Ecology Progress Series, 249, 207-214.
Diaz, R.J. & Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
Diederich, S., 2006. High survival and growth rates of introduced Pacific oysters may cause restrictions on habitat use by native mussels in the Wadden Sea. Journal of Experimental Marine Biology and Ecology, 328 (2), 211-227.
Dixon, P.S. & Irvine, L.M., 1977. Seaweeds of the British Isles. Volume 1 Rhodophyta. Part 1 Introduction, Nemaliales, Gigartinales. London: British Museum (Natural History) London.
Doherty, S.D., Brophy, D. & Gosling, E., 2009. Synchronous reproduction may facilitate introgression in a hybrid mussel (Mytilus) population. Journal of Experimental Marine Biology and Ecology, 378, 1-7.
Ekaratne, S.U.K. & Crisp, D.J., 1984. Seasonal growth studies of intertidal gastropods from shell micro-growth band measurements, including a comparison with alternative methods. Journal of the Marine Biological Association of the United Kingdom, 64, 183-210.
Eno, N.C., Clark, R.A. & Sanderson, W.G. (ed.) 1997. Non-native marine species in British waters: a review and directory. Peterborough: Joint Nature Conservation Committee.
Escapa, M., Isacch, J.P., Daleo, P., Alberti, J., Iribarne, O., Borges, M., Dos Santos, E.P., Gagliardini, D.A. & Lasta, M., 2004. The distribution and ecological effects of the introduced Pacific oyster Crassostrea gigas (Thunberg, 1793) in Northern Patagonia. Journal of Shelfish Research, 23 (3), 765-722.
Essink, K., 1999. Ecological effects of dumping of dredged sediments; options for management. Journal of Coastal Conservation, 5, 69-80.
Evans, R.G., 1948. The lethal temperatures of some common British littoral molluscs. The Journal of Animal Ecology, 17, 165-173.
Ezgeta-Balic, D., Segvic-Bubic, T., Staglicic, N., Lin, Y. P., Bojanic Varezic, D., Grubisic, L. & Briski, E., 2019. Distribution of non-native Pacific oyster Magallana gigas (Thunberg, 1793) along the eastern Adriatic coast. Acta Adriatica, 60 (2), 137-146. DOI https://doi.org/10.32582/aa.60.2.3
Feare, C.J., 1970b. Aspects of the ecology of an exposed shore population of dogwhelks Nucella lapillus. Oecologia, 5, 1-18.
Fletcher, H. & Frid, C.L.J., 1996a. Impact and management of visitor pressure on rocky intertidal algal communities. Aquatic Conservation: Marine and Freshwater Ecosystems, 6, 287-297.
Foster, B.A., 1970. Responses and acclimation to salinity in the adults of some balanomorph barnacles. Philosophical Transactions of the Royal Society of London, Series B, 256, 377-400.
Foster, B.A., 1971b. On the determinants of the upper limit of intertidal distribution of barnacles. Journal of Animal Ecology, 40, 33-48.
Foster, P., Hunt, D.T.E. & Morris, A.W., 1978. Metals in an acid mine stream and estuary. Science of the Total Environment, 9, 75-86.
Frechette, M., Butman, C.A., Geyer, W.R., 1989. The importance of boundary-layer flow in supplying phytoplankton to the benthic suspension feeder, Mytilus edulis L. Limnology and Oceanography, 34, 19-36.
Fretter, V. & Graham, A., 1994. British prosobranch molluscs: their functional anatomy and ecology, revised and updated edition. London: The Ray Society.
Gallagher, M.C., Davenport, J., Gregory, S., McAllen, R. & O'Riordan, R., 2015. The invasive barnacle species, Austrominius modestus: Its status and competition with indigenous barnacles on the Isle of Cumbrae, Scotland. Estuarine, Coastal and Shelf Science, 152, 134-141.
GBNNSS, 2011. Risk assessment for Crassostrea gigas. GB Non-native Species Information Portal, GB Non-native Species Secretariat. Available from: https://www.nonnativespecies.org/assets/Uploads/RA_Crassostrea_gigas_finalpoc.pdf
GBNNSS, 2012. Pacific oyster Magallana gigas. Factsheet. GB Non-native Species Information Portal, [online] GB Non-native Species Secretariat. [Accessed July 2024]. Available from: https://www.nonnativespecies.org/non-native-species/information-portal/view/1013
Gibbs, P.E., Green, J.C. & Pascoe, P.C., 1999. A massive summer kill of the dog-whelk, Nucella lapillus, on the north Cornwall coast in 1995: freak or forerunner? Journal of the Marine Biological Association of the United Kingdom, 79, 103-109.
Glegg, G. A., Hickman, L. & Rowland, S. J., 1999. Contamination of limpets (Patella vulgata) following the Sea Empress oil spill. Marine Pollution Bulletin, 38, 119-125.
Gomes-Filho, J., Hawkins, S., Aquino-Souza, R. & Thompson, R., 2010. Distribution of barnacles and dominance of the introduced species Elminius modestus along two estuaries in South-West England. Marine Biodiversity Records, 3, e58.
Gosling, E.M. (ed.), 1992a. The mussel Mytilus: ecology, physiology, genetics and culture. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25]
Gray, J.S., Wu R.S.-S. & Or Y.Y., 2002. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series, 238, 249-279. DOI https://doi.org/10.3354/meps238249
Grenon, J.F. & Walker, G., 1981. The tenacity of the limpet, Patella vulgata L.: an experimental approach. Journal of Experimental Marine Biology and Ecology, 54, 277-308.
Groenewold, S. & Fonds, M., 2000. Effects on benthic scavengers of discards and damaged benthos produced by the beam-trawl fishery in the southern North Sea. ICES Journal of Marine Science, 57 (5), 1395-1406.
Gyory, J. & Pineda, J., 2011. High-frequency observations of early-stage larval abundance: do storms trigger synchronous larval release in Semibalanus balanoides? Marine Biology, 158 (7), 1581-1589.
Gyory, J., Pineda, J. & Solow, A., 2013. Turbidity triggers larval release by the intertidal barnacle Semibalanus balanoides. Marine Ecology Progress Series, 476, 141-151.
Hansen, B.W., Dolmer, P. & Vismann, B., 2023. Too late for regulatory management on Pacific oysters in European coastal waters? Journal of Sea Research, 191. DOI https://doi.org/10.1016/j.seares.2022.102331
Hartnoll, R.G. & Hawkins, S.J., 1985. Patchiness and fluctuations on moderately exposed rocky shores. Ophelia, 24, 53-63.
Hawkins, A., Smith, R., Bayne, B. & Heral, M., 1996. Novel observations underlying the fast growth of suspension-feeding shellfish in turbid environments: Mytilus edulis. Marine Ecology Progress Series, 131, 179-90
Hawkins, S., 1983. Interactions of Patella and macroalgae with settling Semibalanus balanoides (L.). Journal of Experimental Marine Biology and Ecology, 71 (1), 55-72.
Hawkins, S.J. & Harkin, E., 1985. Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man. Botanica Marina, 28, 223-30.
Hawkins, S.J. & Hartnoll, R.G., 1983. Grazing of intertidal algae by marine invertebrates. Oceanography and Marine Biology: an Annual Review, 21, 195-282.
Hawkins, S.J. & Hartnoll, R.G., 1985. Factors determining the upper limits of intertidal canopy-forming algae. Marine Ecology Progress Series, 20, 265-271.
Hawkins, S.J. & Southward, A.J., 1992. The Torrey Canyon oil spill: recovery of rocky shore communities. In Restoring the Nations Marine Environment, (ed. G.W. Thorpe), Chapter 13, pp. 583-631. Maryland, USA: Maryland Sea Grant College.
Hawkins, S.J., 1981. The influence of Patella grazing on the fucoid/barnacle mosaic on moderately exposed rocky shores. Kieler Meeresforschungen, 5, 537-543.
Hawkins, S.J., Hartnoll, R.G., Kain, J.M. & Norton, T.A., 1992. Plant-animal interactions on hard substrata in the north-east Atlantic. In Plant-animal interactions in the marine benthos (ed. D.M. John, S.J. Hawkins & J.H. Price), pp. 1-32. Oxford: Clarendon Press. [Systematics Association Special Volume, no. 46.]
Hawkins, S.J., Proud, S.V., Spence, S.K. & Southward, A.J., 1994. From the individual to the community and beyond: water quality, stress indicators and key species in coastal systems. In Water quality and stress indicators in marine and freshwater ecosystems: linking levels of organisation (individuals, populations, communities) (ed. D.W. Sutcliffe), 35-62. Ambleside, UK: Freshwater Biological Association.
Hawkins, S.J., Southward, A.J. & Barrett, R.L., 1983. Population structure of Patella vulgata (L.) during succession on rocky shores in southwest England. Oceanologica Acta, Special Volume, 103-107.
Herbert, R. & Hawkins, S., 2006. Effect of rock type on the recruitment and early mortality of the barnacle Chthamalus montagui. Journal of Experimental Marine Biology and Ecology, 334 (1), 96-108.
Herbert, R.J.H., Humphreys, J., Davies, C.J., Roberts, C., Fletcher, S. & Crowe, T.P., 2016. Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodiversity and Conservation, 25 (14), 2835-2865. DOI https://doi.org/10.1007/s10531-016-1209-4
Herbert, R.J.H., Roberts, C., Humphreys, J., & Fletcher, S. 2012. The Pacific oyster (Crassostra gigas) in the UK: economic, legal and environmental issues associated with its cultivation, wild establishment and exploitation. Available from: https://www.daera-ni.gov.uk/publications/pacific-oyster-uk-issues-associated-its-cultivation-wild-establishment-and-exploitation
Hill, S., Burrows, S.J. & Hawkins, S.J., 1998. Intertidal Reef Biotopes (Volume VI). An overview of dynamics and sensitivity characteristics for conservation management of marine Special Areas of Conservation. Oban: Scottish Association for Marine Science (UK Marine SACs Project)., Scottish Association for Marine Science (UK Marine SACs Project). Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/reefbiot.pdf
Hills, J. & Thomason, J., 1998. The effect of scales of surface roughness on the settlement of barnacle (Semibalanus balanoides) cyprids. Biofouling, 12 (1-3), 57-69.
Hily, C., Potin, P. & Floch, J.Y. 1992. Structure of subtidal algal assemblages on soft-bottom sediments - fauna flora interactions and role of disturbances in the Bay of Brest, France. Marine Ecology Progress Series, 85, 115-130.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Holmes, S.P., Walker, G. & van der Meer, J., 2005. Barnacles, limpets and periwinkles: the effects of direct and indirect interactions on cyprid settlement and success. Journal of Sea Research, 53 (3), 181-204.
Holt, T.J., Hartnoll, R.G. & Hawkins, S.J., 1997. The sensitivity and vulnerability to man-induced change of selected communities: intertidal brown algal shrubs, Zostera beds and Sabellaria spinulosa reefs. English Nature, Peterborough, English Nature Research Report No. 234.
Holt, T.J., Jones, D.R., Hawkins, S.J. & Hartnoll, R.G., 1995. The sensitivity of marine communities to man induced change - a scoping report. Countryside Council for Wales, Bangor, Contract Science Report, no. 65.
Holt, T.J., Rees, E.I., Hawkins, S.J. & Seed, R., 1998. Biogenic reefs (Volume IX). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scottish Association for Marine Science (UK Marine SACs Project), 174 pp. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/biogreef.pdf
Hong, J. & Reish, D.J., 1987. Acute toxicity of cadmium to eight species of marine amphipod and isopod crustaceans from southern California. Bulletin of Environmental Contamination and Toxicology, 39, 884-888.
Jenkins, S., Åberg, P., Cervin, G., Coleman, R., Delany, J., Della Santina, P., Hawkins, S., LaCroix, E., Myers, A. & Lindegarth, M., 2000. Spatial and temporal variation in settlement and recruitment of the intertidal barnacle Semibalanus balanoides (L.)(Crustacea: Cirripedia) over a European scale. Journal of Experimental Marine Biology and Ecology, 243 (2), 209-225.
Jenkins, S., Aberg, P., Cervin, G., Coleman, R., Delany, J., Hawkins, S., Hyder, K., Myers, A., Paula, J. & Power, A., 2001b. Population dynamics of the intertidal barnacle Semibalanus balanoides at three European locations: spatial scales of variability. Marine Ecology Progress Series, 217, 207-217.
Jenkins, S.R., Norton, T.A. & Hawkins, S.J., 1999. Settlement and post-settlement interactions between Semibalanus balanoides (L.)(Crustacea: Cirripedia) and three species of fucoid canopy algae. Journal of Experimental Marine Biology and Ecology, 236 (1), 49-67.
JNCC (Joint Nature Conservation Committee), 2022. The Marine Habitat Classification for Britain and Ireland Version 22.04. [Date accessed]. Available from: https://mhc.jncc.gov.uk/
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Joyce, P. W. S., Smyth, D. M., Dick, J. T. A. & Kregting, L. T., 2021. Coexistence of the native mussel, Mytilus edulis, and the invasive Pacific oyster, Crassostrea (Magallana) gigas, does not affect their growth or mortality, but reduces condition of both species. Hydrobiologia, 848 (8), 1859-1871. DOI https://doi.org/10.1007/s10750-021-04558-1
Jørgensen, C.B., 1981. Mortality, growth, and grazing impact on a cohort of bivalve larvae, Mytilus edulis L. Ophelia, 20, 185-192.
Jørgensen, T., 1990. Long-term changes in age at sexual maturity of Northeast Arctic cod (Gadus morhua L.). ICES Journal du Conseil, 46, 235-248.
Kaiser, M.J. & Spencer, B.E., 1994. Fish scavenging behaviour in recently trawled areas. Marine Ecology Progress Series, 112 (1-2), 41-49.
Kautsky, N., 1981. On the trophic role of the blue mussel (Mytilus edulis L.) in a Baltic coastal ecosystem and the fate of the organic matter produced by the mussels. Kieler Meeresforschungen Sonderheft, 5, 454-461.
Kendall, M.A. & Bedford, M.L., 1987. Reproduction and recruitment of the barnacle Chthamalus montagui at Aberystwyth (mid-Wales). Marine Ecology Progress Series, 38, 305-308.
Kendall, M.A., Bowman, R.S., Williamson, P. & Lewis, J.R., 1985. Annual variation in the recruitment of Semibalanus balanoides on the North Yorkshire coast 1969-1981. Journal of the Marine Biological Association of the United Kingdom, 65, 1009-1030.
Kinne, O. (ed.), 1980. Diseases of marine animals. vol. 1. General aspects. Protozoa to Gastropoda. Chichester: John Wiley & Sons.
Kittner, C. & Riisgaard, H.U., 2005. Effect of temperature on filtration rate in the mussel Mytilus edulis: no evidence for temperature compensation. Marine Ecology Progress Series 305: 147-52
Kochmann, J, 2012. Into the Wild Documenting and Predicting the Spread of Pacific Oysters (Crassostrea gigas) in Ireland. PhD Thesis, University College Dublin. Available from: https://www.tcd.ie/research/simbiosys/images/JKPhD.pdf
Kochmann, J., Buschbaum, C., Volkenborn, N. & Reise, K., 2008. Shift from native mussels to alien oysters: differential effects of ecosystem engineers. Journal of Experimental Marine Biology and Ecology, 364 (1), 1-10. DOI https://doi.org/10013/epic.31007.d001
Kochmann, J., O’Beirn, F., Yearsley, J. & Crowe, T.P., 2013. Environmental factors associated with invasion: modelling occurrence data from a coordinated sampling programme for Pacific oysters. Biological Invasions, 15 (10), 2265-2279. DOI https://doi.org/10.1007/s10530-013-0452-9
Kronberg, I., 1988. Structure and adaptation of the fauna in the black zone (littoral fringe) along rocky shores in northern Europe. Marine Ecology Progress Series, 49 (1-2), 95-106.
Landsberg, J.H., 1996. Neoplasia and biotoxins in bivalves: is there a connection? Journal of Shellfish Research, 15, 203-230.
Lejart, M. & Hily, C., 2011. Differential response of benthic macrofauna to the formation of novel oyster reefs (Crassostrea gigas, Thunberg) on soft and rocky substrate in the intertidal of the Bay of Brest, France. Journal of Sea Research, 65 (1), 84-93. DOI https://doi.org/10.1016/j.seares.2010.07.004
Leonard, G.H., Levine, J.M., Schmidt, P.R. & Bertness, M.D., 1998. Flow-driven variation in intertidal community structure in a Maine estuary. Ecology, 79 (4), 1395-1411.
Le Quesne W.J.F. 2005. The response of a protandrous species to exploitation, and the implications for management: a case study with patellid limpets. PhD thesis. University of Southampton, Southampton, United Kingdom.
Lewis, J. & Bowman, R.S., 1975. Local habitat-induced variations in the population dynamics of Patella vulgata L. Journal of Experimental Marine Biology and Ecology, 17 (2), 165-203.
Little, C. & Kitching, J.A., 1996. The Biology of Rocky Shores. Oxford: Oxford University Press.
Little, C., Partridge, J.C. & Teagle, L., 1991. Foraging activity of limpets in normal and abnormal tidal regimes. Journal of the Marine Biological Association of the United Kingdom, 71, 537-554.
Littler, M.M., Martz, D.R. & Littler, D.S., 1983. Effects of recurrent sand deposition on rocky intertidal organisms: importance of substrate heterogeneity in a fluctuating environment. Marine Ecology Progress Series. 11 (2), 129-139.
Livingstone, D.R. & Pipe, R.K., 1992. Mussels and environmental contaminants: molecular and cellular aspects. In The mussel Mytilus: ecology, physiology, genetics and culture, (ed. E.M. Gosling), pp. 425-464. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25]
Long, J.D., Cochrane, E. & Dolecal, R., 2011. Previous disturbance enhances the negative effects of trampling on barnacles. Marine Ecology Progress Series, 437, 165-173.
Loo, L-O., 1992. Filtration, assimilation, respiration and growth of Mytilus edulis L. at low temperatures. Ophelia 35: 123-31
Loosanoff, V.L., 1962. Effects of turbidity on some larval and adult bivalves. Proceedings of the Gulf and Caribbean Fisheries Institute, 14, 80-95.
Maggs, C.A. & Hommersand, M.H., 1993. Seaweeds of the British Isles: Volume 1 Rhodophycota Part 3A Ceramiales. London: Natural History Museum, Her Majesty's Stationary Office.
Marchan, S., Davies, M.S., Fleming, S. & Jones, H.D., 1999. Effects of copper and zinc on the heart rate of the limpet Patella vulgata (L.) Comparative Biochemistry and Physiology, 123A, 89-93.
Marshall, D.J. & McQuaid, C.D., 1989. The influence of respiratory responses on the tolerance to sand inundation of the limpets Patella granularis L.(Prosobranchia) and Siphonaria capensis Q. et G.(Pulmonata). Journal of Experimental Marine Biology and Ecology, 128 (3), 191-201.
Marshall, D.J. & McQuaid, C.D., 1993. Effects of hypoxia and hyposalinity on the heart beat of the intertidal limpets Patella granvlaris (Prosobranchia) and Siphonaria capensis (Pulmonata). Comparative Biochemistry and Physiology Part A: Physiology, 106 (1), 65-68
McGrorty, S., Clarke, R.T., Reading, C.J. & Goss, C.J.D., 1990. Population dynamics of the mussel Mytilus edulis: density changes and regulation of the population in the Exe Estuary, Devon. Marine Ecology Progress Series, 67, 157-169.
McKay, D.W., 1994. Aulacomya ater (Mollina, 1782) [Mollusca: Pelecypoda] collected from the Moray Firth. Porcupine Newsletter, 5, 23.
McKinstry K. & Jensen A., 2013. Distribution, abundance and temporal variation of the Pacific oyster, Crassostrea gigas in Poole Harbour. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/313003/fcf-oyster.pdf
McLusky, D.S., Bryant, V. & Campbell, R., 1986. The effects of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanography and Marine Biology: an Annual Review, 24, 481-520.
Mieszkowska, N., Burrows, M.T., Pannacciulli, F.G. & Hawkins, S.J., 2014. Multidecadal signals within co-occurring intertidal barnacles Semibalanus balanoides and Chthamalus spp. linked to the Atlantic Multidecadal Oscillation. Journal of Marine Systems, 133, 70-76.
Monterosso, B., 1930. Studi cirripedologici. VI. Sul comportamento di Chthamalus stellatus in diverse condizioni sperimentali. Atti Accad. Naz. Lincei Rc., 9, 501-504.
Moore, J., 1997. Rocky shore transect monitoring in Milford Haven, October 1996. Impacts of the Sea Empress oil spill. Countryside Council for Wales Sea Empress Contract Report, 241, 90pp.
Moore, P.G., 1977a. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanography and Marine Biology: An Annual Review, 15, 225-363.
Morgan, A., Slater, M., Mortimer, N., McNie, F., Singfield, C., Bailey, L., Covey, R., McNair, S., Waddell, C., Crundwell, R., Gall, A., Selley, H. & Packer, N., 2021. Partnership led strategy to monitor and manage spread of Pacific oyster populations in south Devon and Cornwall. Natural England Research Reports, NERR100. Natural England Research Reports, NERR100, Natural England, Truro, Cornwall, 258 pp. Available from: https://publications.naturalengland.org.uk/publication/4889256448491520#:~:text=Between 2017 and 2020, volunteers,method of controlling population expansion.
Mrowicki, R.J., Maggs, C.A. & O'Connor, N.E., 2014. Does wave exposure determine the interactive effects of losing key grazers and ecosystem engineers? Journal of Experimental Marine Biology and Ecology, 461 (0), 416-424.
Myrand, B., Guderley, H. & Himmelman, J.H., 2000. Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence. Marine Ecology Progress Series 197: 193-207
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Nehls, G., Diederich, S., Thieltges, David W. & Strasser, M., 2006. Wadden Sea mussel beds invaded by oysters and slipper limpets: competition or climate control? Helgoland Marine Research, 60 (2), 135-143. DOI https://doi.org/10.1007/s10152-006-0032-9
Newell, R.C., 1979. Biology of intertidal animals. Faversham: Marine Ecological Surveys Ltd.
Norton, T.A., 1992. Dispersal by macroalgae. British Phycological Journal, 27, 293-301.
O'Brien, P.J. & Dixon, P.S., 1976. Effects of oils and oil components on algae: a review. British Phycological Journal, 11, 115-142.
O'Riordan, R.M., Myers, A.A. & Cross, T.F., 1992. Brooding in the intertidal barnacles Chthamalus stellatus (Poli) and Chthamalus montagui (Southward) in south-western Ireland. Journal of Experimental Marine Biology and Ecology, 164, 135-145.
O'Riordan, R.M., Myers, A.A. & Cross, T.F., 1995. The reproductive cycles of Chthamalus stellatus (Poli) and Chthamalus montagui (Southward) in south-western Ireland. Journal of Experimental Marine Biology and Ecology, 190, 17-38.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Padilla, D.K., 2010. Context-dependent impacts of a non-native ecosystem engineer, the Pacific Oyster Crassostrea gigas. Integrative and Comparative Biology, 50 (2), 213-225. DOI https://doi.org/10.1093/icb/icq080
Patel, B. & Crisp, D. J., 1960. The influence of temperature on the breeding and the moulting activities of some warm-water species of operculate barnacles. Journal of the Marine Biological Association of the United Kingdom, 36, 667-680.
Petpiroon, S. & Dicks, B., 1982. Environmental effects (1969 to 1981) of a refinery effluent discharged into Littlewick Bay, Milford Haven. Field Studies, 5, 623-641.
Petraitis, P.S. & Dudgeon, S.R., 2005. Divergent succession and implications for alternative states on rocky intertidal shores. Journal of Experimental Marine Biology and Ecology, 326 (1), 14-26. DOI https://doi.org/10.1016/j.jembe.2005.05.013
Petraitis, P.S., Rhile, E.C. & Dudgeon, S., 2003. Survivorship of juvenile barnacles and mussels: spatial dependence and the origin of alternative communities. Journal of Experimental Marine Biology and Ecology, 293 (2), 217-236.
Pieters, H., Klutymans, J.H., Zandee, D.I. & Cadee, G.C., 1980. Tissue composition and reproduction of Mytilus edulis dependent upon food availability. Netherlands Journal of Sea Research, 14, 349-361.
Povey, A. & Keough, M.J., 1991. Effects of trampling on plant and animal populations on rocky shores. Oikos, 61: 355-368.
Prendergast, G.S., Zurn, C.M., Bers, A.V., Head, R.M., Hansson, L.J. & Thomason, J.C., 2009. The relative magnitude of the effects of biological and physical settlement cues for cypris larvae of the acorn barnacle, Semibalanus balanoides L. Biofouling, 25 (1), 35-44.
Purchon, R.D., 1937. Studies on the biology of the Bristol Channel. Proceedings of the Bristol Naturalists' Society, 8, 311-329.
Raffaelli, D.G. & Hawkins, S.J., 1999. Intertidal Ecology 2nd edn.. London: Kluwer Academic Publishers.
Rainbow, P.S., 1984. An introduction to the biology of British littoral barnacles. Field Studies, 6, 1-51.
Ramsay, K., Kaiser, M.J. & Hughes, R.N. 1998. The responses of benthic scavengers to fishing disturbance by towed gears in different habitats. Journal of Experimental Marine Biology and Ecology, 224, 73-89.
Read, K.R.H. & Cumming, K.B., 1967. Thermal tolerance of the bivalve mollusc Modiolus modiolus (L.), Mytilus edulis (L.) and Brachiodontes demissus (Dillwyn). Comparative Biochemistry and Physiology, 22, 149-155.
Ribeiro, P.A., Xavier, R., Santos, A.M. & Hawkins, S.J., 2009. Reproductive cycles of four species of Patella (Mollusca: Gastropoda) on the northern and central Portuguese coast. Journal of the Marine Biological Association of the United Kingdom, 89 (06), 1215-1221.
Rognstad, R.L., Wethey, D.S. & Hilbish, T.J., 2014. Connectivity and population repatriation: limitations of climate and input into the larval pool. Marine Ecology Progress Series, 495, 175-183.
Ruesink, J.L., Lenihan, H.S., Trimble, A.C., Heiman, K.W., Micheli, F., Byers, J.E. & Kay, M.C., 2005. Introduction of Non-Native Oysters: Ecosystem Effects and Restoration Implications. Annual Review of Ecology, Evolution, and Systematics, 36 (Volume 36, 2005), 643-689. DOI https://doi.org/10.1146/annurev.ecolsys.36.102003.152638
Safriel, U.N., Erez, N. & Keasar, T., 1994. How do limpets maintain barnacle-free submerged artificial surfaces? Bulletin of Marine Science, 54 (1), 17-23.
Sanford, E., Bermudez, D., Bertness, M.D. & Gaines, S.D., 1994. Flow, food supply and acorn barnacle population dynamics. Marine Ecology Progress Series, 104, 49-49.
Schiel, D.R. & Foster, M.S., 1986. The structure of subtidal algal stands in temperate waters. Oceanography and Marine Biology: an Annual Review, 24, 265-307.
Schiel, D.R. & Taylor, D.I., 1999. Effects of trampling on a rocky intertidal algal assemblage in southern New Zealand. Journal of Experimental Marine Biology and Ecology, 235, 213-235.
Seapy , R.R. & Littler, M.M., 1982. Population and Species Diversity Fluctuations in a Rocky Intertidal Community Relative to Severe Aerial Exposure and Sediment Burial. Marine Biology, 71, 87-96.
Seed, R. & Suchanek, T.H., 1992. Population and community ecology of Mytilus. In The mussel Mytilus: ecology, physiology, genetics and culture, (ed. E.M. Gosling), pp. 87-169. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25.]
Seed, R., 1969b. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores 2. Growth and mortality. Oecologia, 3, 317-350.
Seed, R., 1996. Patterns of biodiversity in the macro-invertebrate fauna associated with mussel patches on rocky shores. Journal of the Marine Biological Association of the United Kingdom, 76, 203-210.
Shanks, A.L. & Wright, W.G., 1986. Adding teeth to wave action- the destructive effects of wave-bourne rocks on intertidal organisms. Oecologia, 69 (3), 420-428.
Shumway, S.E., 1990. A review of the effects of algal blooms on shellfish and aquaculture. Journal of the World Aquaculture Society, 21, 65-104.
Shumway, S.E., 1992. Mussels and public health. In The mussel Mytilus: ecology, physiology, genetics and culture, (ed. E. Gosling), pp. 511-542. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25]
Smith, B.S., 1980. The estuarine mud snail, Nassarius obsoletus: abnormalities in the reproductive system. Journal of Molluscan Studies, 46, 247-256.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Southward, A., 1978. Marine life and Amoco Cadiz. New Scientist, 79, 174-176
Southward, A.J. & Crisp, D.J., 1956. Fluctuations in the distribution and abundance of intertidal barnacles. Journal of the Marine Biological Association of the United Kingdom, 35, 211-229.
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Southward, A.J., 1955. On the behaviour of barnacles. I. The relation of cirral and other activities to temperature. Journal of the Marine Biological Association of the United Kingdom, 34, 403-432.
Southward, A.J., 1964. Limpet grazing and the control of vegetation on rocky shores. In Grazing in Terrestrial and Marine Environments, British Ecological Society Symposium No. 4 (ed. D.J. Crisp), 265-273.
Southward, A.J., Hawkins, S.J. & Burrows, M.T., 1995. Seventy years observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. Journal of Thermal Biology, 20, 127-155.
Spagnolo, A., Auriemma, R., Bacci, T., Balkovic, I., Bertasi, F., Bolognini, L., Cabrini, M., Cilenti, L., Cuicchi, C., Cvitkovic, I., Despalatovic, M., Grati, F., Grossi, L., Jaklin, A., Lipej, L., Markovic, O., Mavric, B., Mikac, B., Nasi, F., Nerlovic, V., Pelosi, S., Penna, M., Petovic, S., Punzo, E., Santucci, A., Scirocco, T., Strafella, P., Trabucco, B., Travizi, A. & Zuljevic, A., 2019. Non-indigenous macrozoobenthic species on hard substrata of selected harbours in the Adriatic Sea. Marine Pollution Bulletin, 147, 150-158. DOI https://doi.org/10.1016/j.marpolbul.2017.12.031
Spencer, B. E., Edwards, D. B., Kaiser, M. J. & Richardson, C. A., 1994. Spatfalls of the non-native Pacific oyster, Crassostrea gigas, in British waters. Aquatic Conservation: Marine and Freshwater Ecosystems, 4 (3), 203-217. DOI https://doi.org/10.1002/aqc.3270040303
Suchanek, T.H., 1978. The ecology of Mytilus edulis L. in exposed rocky intertidal communities. Journal of Experimental Marine Biology and Ecology, 31, 105-120.
Suchanek, T.H., 1985. Mussels and their role in structuring rocky shore communities. In The Ecology of Rocky Coasts: essays presented to J.R. Lewis, D.Sc., (ed. P.G. Moore & R. Seed), pp. 70-96.
Suchanek, T.H., 1993. Oil impacts on marine invertebrate populations and communities. American Zoologist, 33, 510-523. DOI https://doi.org/10.1093/icb/33.6.510
Svåsand, T., Crosetti, D., García-Vázquez, E. & Verspoor, E., 2007. Genetic impact of aquaculture activities on native populations. Genimpact final scientific report (EU contract n. RICA-CT-2005-022802).
Sweet, N.S. & Sewell, J. 2014. Asian shore crab, Hemigrapsus sanguineus. Great Britain Non-native Species Secretariat. [cited 16/06/2015]. Available from: <http://www.nonnativespecies.org
Teschke, K., Karez, R., Schubert, P. R. & Beermann, J., 2020. Colonisation success of introduced oysters is driven by wave-related exposure. Biological Invasions, 22 (7), 2121-2127. DOI https://doi.org/10.1007/s10530-020-02246-0
Thompson, G.B., 1980. Distribution and population dynamics of the limpet Patella vulgata in Bantry Bay. Journal of Experimental Marine Biology and Ecology, 45, 173-217.
Thompson, I., Richardson, C., Seed R. & Walker G., 2000. Quantification of mussel (Mytilus edulis) growth from power station cooling waters in response to chlorination procedures. Biofouling, 16(1), 1-15.
Thompson, R.C., Olsen, Y., Mitchell, R.P., Davis, A., Rowland, S.J., John, A.W., McGonigle, D. & Russell, A.E., 2004. Lost at sea: where is all the plastic? Science, 304 (5672), 838-838.
Tighe-Ford, D., 1967. Possible mechanism for the endocrine control of breeding in a cirripede. Nature, 216, 920-921.
Tillin, H.M., Kessel, C., Sewell, J., Wood, C.A. & Bishop, J.D.D., 2020. Assessing the impact of key Marine Invasive Non-Native Species on Welsh MPA habitat features, fisheries and aquaculture. NRW Evidence Report. Report No: 454. Natural Resources Wales, Bangor, 260 pp. Available from https://naturalresourceswales.gov.uk/media/696519/assessing-the-impact-of-key-marine-invasive-non-native-species-on-welsh-mpa-habitat-features-fisheries-and-aquaculture.pdf
Trager, G. C., Hwang, J. S., & Strickler, J. R. 1990. Barnacle suspension-feeding in variable flow. Marine Biology, 105(1), 117-127.
Troost, K., 2010. Causes and effects of a highly successful marine invasion: case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. Journal of Sea Research, 64 (3), 145-165. DOI https://doi.org/10.1016/j.seares.2010.02.004
Tsuchiya, M. & Nishihira, M., 1985. Islands of Mytilus as a habitat for small intertidal animals: effect of island size on community structure. Marine Ecology Progress Series, 25, 71-81.
Tsuchiya, M. & Nishihira, M., 1986. Islands of Mytilus edulis as a habitat for small intertidal animals: effect of Mytilus age structure on the species composition of the associated fauna and community organization. Marine Ecology Progress Series, 31, 171-178.
Tyler-Walters, H. & Arnold, C., 2008. Sensitivity of Intertidal Benthic Habitats to Impacts Caused by Access to Fishing Grounds. Report to Cyngor Cefn Gwlad Cymru / Countryside Council for Wales from the Marine Life Information Network (MarLIN) [Contract no. FC 73-03-327], Marine Biological Association of the UK, Plymouth, 48 pp. Available from: www.marlin.ac.uk/publications
Vadas, R.L., Johnson, S. & Norton, T.A., 1992. Recruitment and mortality of early post-settlement stages of benthic algae. British Phycological Journal, 27, 331-351.
Van de Werfhorst, L.C. & Pearse J.S., 2007. Trampling in the rocky intertidal of central California: a follow-up study. Bulletin of Marine Science, 81(2), 245-254.
Wethey, D.S., 1985. Catastrophe, Extinction, and Species Diversity: A Rocky Intertidal Example. Ecology, 66 (2), 445-456.
Wethey, D.S., 1984. Sun and shade mediate competition in the barnacles Chthamalus and Semibalanus: a field experiment. The Biological Bulletin, 167 (1), 176-185.
Wethey, D.S., Woodin, S.A., Hilbish, T.J., Jones, S.J., Lima, F.P. & Brannock, P.M., 2011. Response of intertidal populations to climate: effects of extreme events versus long term change. Journal of Experimental Marine Biology and Ecology, 400 (1), 132-144.
Whitehouse, J., Coughlan, J., Lewis, B., Travade, F. & Britain, G., 1985. The control of biofouling in marine and estuarine power stations: a collaborative research working group report for use by station designers and station managers. Central Electricity Generating Board
Widdows J., Lucas J.S., Brinsley M.D., Salkeld P.N. & Staff F.J., 2002. Investigation of the effects of current velocity on mussel feeding and mussel bed stability using an annular flume. Helgoland Marine Research, 56(1), 3-12.
Widdows, J. & Donkin, P., 1992. Mussels and environmental contaminants: bioaccumulation and physiological aspects. In The mussel Mytilus: ecology, physiology, genetics and culture, (ed. E.M. Gosling), pp. 383-424. Amsterdam: Elsevier Science Publ. [Developments in Aquaculture and Fisheries Science, no. 25]
Widdows, J., 1991. Physiological ecology of mussel larvae. Aquaculture, 94, 147-163.
Widdows, J., Donkin, P., Brinsley, M.D., Evans, S.V., Salkeld, P.N., Franklin, A., Law, R.J. & Waldock, M.J., 1995. Scope for growth and contaminant levels in North Sea mussels Mytilus edulis. Marine Ecology Progress Series, 127, 131-148.
Wood, L. E., Silva, T. A. M., Heal, R., Kennerley, A., Stebbing, P., Fernand, L. & Tidbury, H. J., 2021. Unaided dispersal risk of Magallana gigas into and around the UK: combining particle tracking modelling and environmental suitability scoring. Biological Invasions, 23 (6), 1719-1738. DOI https://doi.org/10.1007/s10530-021-02467-x
Wrange, Anna-Lisa, Valero, Johanna, Harkestad, Lisbeth S., Strand, Øivind, Lindegarth, Susanne, Christensen, Helle Torp, Dolmer, Per, Kristensen, Per Sand & Mortensen, Stein, 2010. Massive settlements of the Pacific oyster, Crassostrea gigas, in Scandinavia. Biological Invasions, 12 (5), 1145-1152. DOI https://doi.org/10.1007/s10530-009-9535-z
Young, G.A., 1985. Byssus thread formation by the mussel Mytilus edulis: effects of environmental factors. Marine Ecology Progress Series, 24, 261-271.
Zandee, D.I., Holwerda, D.A., Kluytmans, J.H. & De Zwaan, A., 1986. Metabolic adaptations to environmental anoxia in the intertidal bivalve mollusc Mytilus edulis L. Netherlands Journal of Zoology, 36(3), 322-343.
Zwerschke, N., Hollyman, P.R., Wild, R., Strigner, R., Turner, J.R. & King, J.W., 2018. Limited impact of an invasive oyster on intertidal assemblage structure and biodiversity: the importance of environmental context and functional equivalency with native species. Marine Biology, 165 (5), 89. DOI https://doi.org//10.1007/s00227-018-3338-7
Citation
This review can be cited as:
Last Updated: 01/08/2024