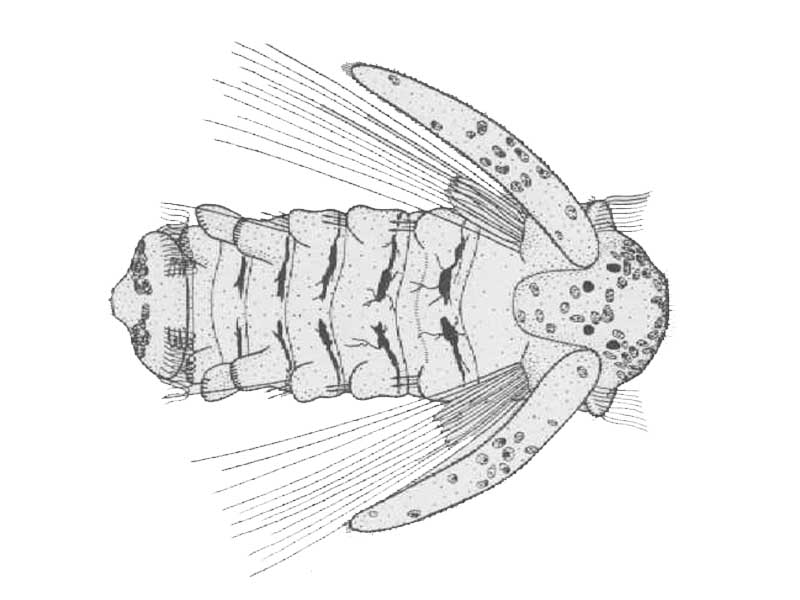
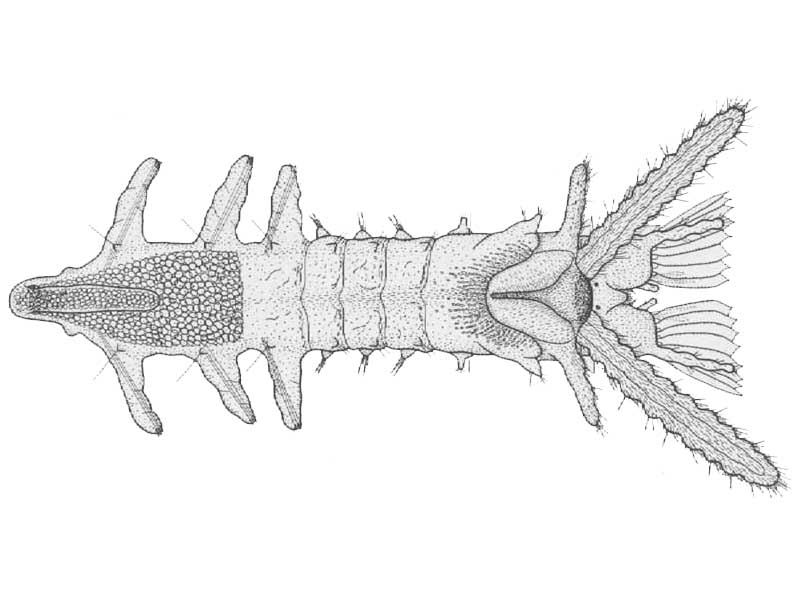
Honeycomb worm (Sabellaria alveolata)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Angus Jackson | Refereed by | Prof. Steve J. Hawkins |
| Authority | (Linnaeus, 1767) | ||
| Other common names | - | Synonyms | - |
Summary
Description
A frequently gregarious segmented worm that builds tubes from sand or shell fragments. Found intertidally (although occasionally subtidally) in exposed areas. Tubes often densely aggregated forming a honey comb pattern. May form large reefs up to several metres across and a metre deep.
Recorded distribution in Britain and Ireland
In Britain, most abundant on the south and west coasts with isolated records form the south east and east coasts. The northern limit is the Outer Hebrides. It is also found on south, west and north coasts of Ireland.
Global distribution
Mediterranean, north Atlantic south to Morocco. The British Isles form the northern limits of the distribution in the north east Atlantic
Habitat
Found on hard substrata on exposed, open coasts with moderate to considerable water movement where sand is available for tube building. Typically on the bottom third of the shoreline but also in the shallow sub-tidal.
Depth range
MTL - 10 metresIdentifying features
- Usually forms sheets or reefs.
- Inhabits tube made from coarse, cemented sand or shell grains.
- Thorax with three pairs of flattened chaetal sheaths.
- Inner and middle rows of opercular chaetae with asymmetrically angular spines pointing distally and transversely respectively.
Additional information
At low densities tubes are attached to the substratum along the entire length but at greater densities competition for space results in the tubes overlapping and may cause the tubes to be built outwards, away from the substrate. Tube colour varies according to the colour of sand grains
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Annelida | Segmented worms e.g. ragworms, tubeworms, fanworms and spoon worms |
| Class | Polychaeta | Bristleworms, e.g. ragworms, scaleworms, paddleworms, fanworms, tubeworms and spoon worms |
| Family | Sabellariidae | |
| Genus | Sabellaria | |
| Authority | (Linnaeus, 1767) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 3-4 cm | ||
| Male size at maturity | |||
| Female size range | Small-medium(3-10cm) | ||
| Female size at maturity | |||
| Growth form | Tubicolous | ||
| Growth rate | 12 cm/year | ||
| Body flexibility | |||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Active suspension feeder | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Seston | ||
| Sociability | Gregarious | ||
| Environmental position | Epifaunal | ||
| Dependency | Independent. | ||
| Supports | Substratum a variety of associated fauna and flora depending on form and age of colony: particularly mussels, barnacles and ephemeral algae | ||
| Is the species harmful? | No | ||
Biology information
The size listed refers to individual worms. It is typically gregarious, forming colonies of sheets, hummocks or reefs. In Cornwall, their tubes are up to 20 cm in length and around 5 mm in diameter at the external opening. Each tube has an additional porch over the opening. In northern France, the tubes were reported to grow in length at 12 cm/year. This species appears to be favoured by elevated winter temperatures associated with cooling water discharges (Bamber & Irving, 1997) but growth is inhibited below 5°C. The communities associated with Sabellaria alveolata are not particularly remarkable being species poor on young dense reefs while up to 38 species have been recorded on older reefs. Honeycomb worm aggregations that bind together mobile cobbles increase heterogeneity.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast |
| Biological zone preferences | Lower eulittoral, Mid eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Bedrock, Cobbles, Large to very large boulders, Pebbles, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed, Very exposed |
| Salinity preferences | Full (30-40 psu) |
| Depth range | MTL - 10 metres |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
The honeycomb worm appears to be absent from many exposed peninsulas, probably due to the effects of water movement on recruitment. Although a hard substratum is required for attachment, there needs to be adequate sand or small shell particles from which to construct the tubes. It is typically found in the low intertidal but occasionally found subtidally (e.g. in the Severn estuary). It typically colonizes bedrock or large boulders but in some sites binds together small cobbles in a complex with mussels. It has a strong settlement preference for adult tubes or sites currently or previously used by the species.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | 100,000-1,000,000 |
| Generation time | Insufficient information |
| Age at maturity | In good conditions worms mature within the first year. |
| Season | July - July |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 1-6 months |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
Most individuals have a lifespan of three to five years but there are records of seven and even nine year old individuals. Sabellaria alveolata reefs undergo cycles of development and decay over a period of a few years. Although individual reefs come and go, areas that are good for Sabellaria alveolata tend to remain so. Spawning occurs each July but actual recruitment levels vary considerably from year to year. Larvae spend between 6 weeks and 6 months in the plankton.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceThe species is fixed to the substratum so substratum removal will cause mortality. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take several years. | High | Moderate | Moderate | Low |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceTolerant to burial under sand for up to several weeks. Feeding and growth will be curtailed. Depending on timing this may interfere with reproduction. Recoverability is almost immediate (Wilson, 1971). | Low | Immediate | Not sensitive | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceTube growth is dependent on the presence of suspended particles hence a reduction in siltation may hinder tube construction. An increase in siltation may facilitate tube building but clog up feeding apparatus. Recovery occurs when the population is able to recommence feeding and growing. | Low | Very high | Very Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceSpecies is typically intertidal and so is regularly exposed to drying influences. When exposed to the air the worm retracts into its tube and closes the operculum over the entrance reducing evaporation. Changes in desiccation for the period of a year may cause some of the population to die. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA reduction in the amount of time spent under water could cause a proportion of a colony to die due to restricted feeding. The species also occurs subtidally so a decrease in emergence time will have no effect. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceThe species inhabits areas with high water flow so an increase in rate is likely to have little effect. A reduction of water flow by two categories is likely to cause exposure to conditions outside the normal range for the species. This may be sufficient to reduce availability of suspended particles, hindering growth and repair and feeding. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceThe species is typically intertidal. Intolerance assessment is in relation to short term acute temperature change. Growth inhibited below 5 degrees C. Damaged or killed by frost. Long term slight increase in temperature is unlikely to have any effect on British populations as global distribution extends South to Morocco. Long term slight decrease in temperature may reduce viability of populations. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceNo reliance on visual sense for feeding, reproducing etc. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceRequires sufficient water action to suspend coarse sand particles in order to build tubes and so is found in quite exposed areas. Most colonies die through eventual break up by wave action. Increased exposure will result in potentially shorter colony life. Reduced exposure may mean the population exists outside of its preferred conditions with insufficient water action to provide sand particles or food. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceThe species is unlikely to respond to noise vibrations | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceMost polychaetes have photoreceptors but the species is probably unable to resolve moving objects. The worms may retract into tube on disturbance. Whether this is through light detection or mechanical stimulus is uncertain. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion through trampling can affect this species but it is surprisingly tolerant (Cunningham et al., 1984). The main cause of colony destruction is through wave action. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement, as this is the preferred substratum (Wilson 1929). Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take several years. | Intermediate | High | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceWorms are not able to rebuild tubes if removed from them (Wilson 1929). | High | Moderate | Moderate | High |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceAlthough the larvae are potentially very intolerant of some oil dispersants it is unlikely that Sabellaria alveolata has any special intolerance to chemicals (Holt et al., 1998). | No information | No information | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceSpecies only occurs in fully marine environment, however, as it frequently occurs in the intertidal, it must be able to tolerate some reduced salinity caused by precipitation run off. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceCole et al. (1999) suggest possible adverse effects on marine species below 4 mg/l and probable adverse effects below 2 mg/l. There is no information about Sabellaria alveolata tolerance to changes in oxygenation. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Very low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficientinformation | No information | No information | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceExtraction by bait digging is a possibility. Automatically assessed as intermediate assuming 50 percent removal. Variability in recruitment (dependent on suitable environmental conditions) means that recovery could take a few years. The presence of some remaining adults will assist in larval settlement as this is the preferred substratum (Wilson 1929). | Intermediate | High | Low | Low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceBait collection occurs in Portugal for crabs etc. living in the gaps between worm tubes. | Intermediate | High | Low | Low |
Additional information
Importance review
Policy/legislation
| Designation | Support |
|---|
Status
| National (GB) importance | Not rare or scarce | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Few crevice fauna are associated with reefs. The main colonizing species are epifaunal. Fishermen occasionally dig out the worms from reefs for use as bait.
Well-established reefs can exclude other organisms such as weeds, barnacles and limpets from the substratum. Sabellaria alveolata reefs are well represented in candidate SACs. Actively growing reefs can out-compete all other littoral species for space.
Bibliography
Bamber, R.N. & Irving, P.W., 1997. The differential growth of Sabellaria alveolata (L.) reefs at a power station outfall. Polychaete Research, 17, 9-14.
Campbell, A., 1994. Seashores and shallow seas of Britain and Europe. London: Hamlyn.
Cunningham, P.N., Hawkins, S.J., Jones, H.D. & Burrows, M.T., 1984. The geographical distribution of Sabellaria alveolata (L.) in England, Wales and Scotland, with investigations into the community structure of and the effects of trampling on Sabellaria alveolata colonies. Nature Conservancy Council, Peterborough, Contract Report no. HF3/11/22., University of Manchester, Department of Zoology.
Gruet, Y. & Lassus, P., 1983. Contribution a l'etude de la biologie reproductive d'une population naturelle de l'Annelide Polychete, Sabellaria alveolata (Linnaeus). Annals of the Institute of Oceanography, Monaco, 59, 127 - 140.
Gruet, Y., 1985. Recherches sur l'é cologie des ré cifs d'hermelles édifiés par l'annélide polychète Sabellaria alveolata (Linné). Journal de Recherche Oceanographique, 10, 32-35.
Gruet, Y., 1986. Spatio-temporal changes of sabellarian reefs built by the sedentary polychaete Sabellaria alveolata (Linnaeus) Marine Ecology, Pubblicazioni della Stazione Zoologica di Napoli I, 7, 303-319.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Holt, T.J., Rees, E.I., Hawkins, S.J. & Seed, R., 1998. Biogenic reefs (Volume IX). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scottish Association for Marine Science (UK Marine SACs Project), 174 pp. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/biogreef.pdf
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Pawlik, J.R., 1968. Larval settlement and metamorphosis of gregarious sabellariid polychaetes, Sabellaria alveolata compared with Phragmatopoma californica. Journal of the Marine Biological Association of the United Kingdom, 68, 101-124.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Wilson, D.P., 1929. The larvae of the British sabellarians. Journal of the Marine Biological Association of the United Kingdom, 16, 221-269.
Wilson, D.P., 1968. Some aspects of the development of the eggs and larvae of Sabellaria alveolata (L.). Journal of the Marine Biological Association of the United Kingdom, 48, 367-86.
Wilson, D.P., 1969. The honey comb worm. Sea Frontiers, 15, 322-29.
Wilson, D.P., 1970a. Additional observations on larval growth and settlement of Sabellaria alveolata. Journal of the Marine Biological Association of the United Kingdom, 50, 1-32.
Wilson, D.P., 1971. Sabellaria colonies at Duckpool, North Cornwall 1961 - 1970 Journal of the Marine Biological Association of the United Kingdom, 54, 509-580.
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Bristol Regional Environmental Records Centre, 2017. BRERC species records within last 15 years. Occurrence dataset: https://doi.org/10.15468/vntgox accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-22
South East Wales Biodiversity Records Centre, 2018. SEWBReC Worms (South East Wales). Occurrence dataset: https://doi.org/10.15468/5vh0w8 accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Citation
This review can be cited as:
Last Updated: 29/04/2008