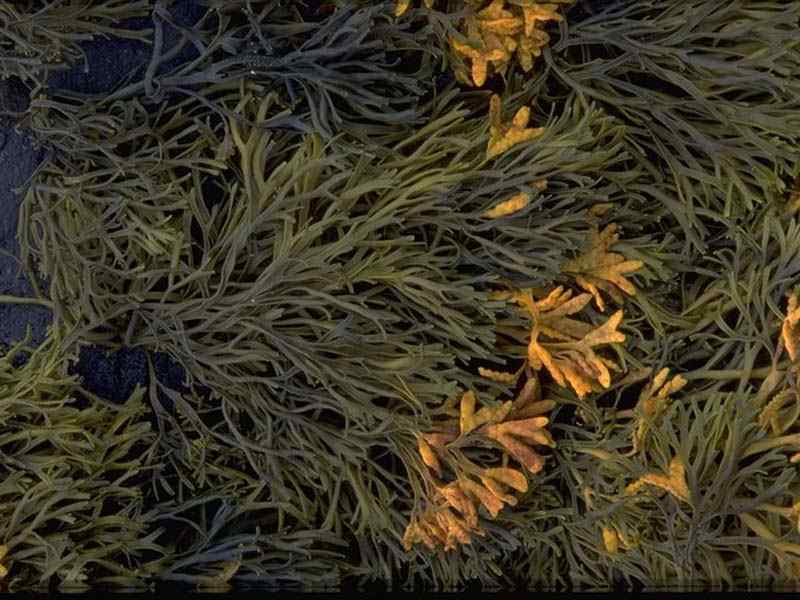
Channelled wrack (Pelvetia canaliculata)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nicola White | Refereed by | Dr Dagmar Stengel |
| Authority | (Linnaeus) Decaisne & Thuret, 1845 | ||
| Other common names | - | Synonyms | - |
Summary
Description
A common brown seaweed found high on the shore. It is very tolerant of desiccation surviving up to 8 days out of the water. Pelvetia canaliculata lives for about 4 years and grows up to 15 cm long. The fronds of the algae are curled longitudinally forming a channel.
Recorded distribution in Britain and Ireland
All coasts of Britain and IrelandGlobal distribution
Norway, Iceland, UK, Ireland, Atlantic coast of France, Spain and Portugal.Habitat
Pelvetia canaliculata grows attached to hard substrata on the upper shore. It is found in a band above Fucus spiralis and can tolerate ultra sheltered to moderately exposed conditions.Depth range
Not relevantIdentifying features
- Frond curled longitudinally to form a distinct channel.
- Without midrib or airbladders.
- Reproductive bodies at ends of branches.
- Dichotomously branched.
Additional information
Pelvetia canaliculata has an obligate endophytic fungus Mycosphaerella acophylli (Ascomycetes).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Ochrophyta | Brown and yellow-green seaweeds |
| Class | Phaeophyceae | |
| Order | Fucales | |
| Family | Fucaceae | |
| Genus | Pelvetia | |
| Authority | (Linnaeus) Decaisne & Thuret, 1845 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | Up to 15 cm | ||
| Male size at maturity | 4 cm | ||
| Female size range | 4 cm | ||
| Female size at maturity | |||
| Growth form | Shrub | ||
| Growth rate | 3-4cm/year | ||
| Body flexibility | |||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Autotroph | ||
| Diet/food source | Photoautotroph | ||
| Typically feeds on | |||
| Sociability | Solitary | ||
| Environmental position | Epifloral | ||
| Dependency | Independent. | ||
| Supports | No information | ||
| Is the species harmful? | No | ||
Biology information
Pelvetia canaliculata is very tolerant of desiccation. It may spend up to 90 percent of the time out of the water and can tolerate 65 percent water loss. The species can photosynthesise when exposed to air but may suffer nutrient stress as it can only obtain nutrients when submerged. The species supports an impoverished fauna due to the harsh physical conditions on the upper shore. A few species of wandering isopods and amphipods may be found sheltering underneath the fronds at low tide.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Strait or Sound, Sea loch or Sea lough, Ria or Voe |
| Biological zone preferences | Lower littoral fringe |
| Substratum / habitat preferences | Bedrock, Cobbles, Large to very large boulders, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Moderately exposed, Sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | Not relevant |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
- Pelvetia canaliculata is the highest living fucoid on the shore. The upper limits of the species distribution are controlled by its physiological tolerances, whereas it's lower limits on the shore are controlled by its ability to compete with Fucus spiralis. Pelvetia canaliculata is capable of growing further down the shore but it is out-competed by faster growing species such as Fucus spiralis and is also heavily grazed. However, this is one of the few algae which requires regular aerial exposure to survive and prolonged submersion, such as in rockpools actually kills the algae.
- In moderately exposed conditions Pelvetia canaliculata is capable of growing above the high water mark, where it is supplied with water through spray and waves. In sheltered conditions it grows further down the shore where it will be immersed by spring tides but often not covered by neaps. It has been estimated that some plants spend up to 90 percent of their time out of the water (Fish & Fish, 1996).
- Pelvetia canaliculata lives in some lower salinity sites although the balance between the algae and the obligate endophytic fungus Mycosphaerella ascophylli seems to be affected (D. Stengel pers. comm.)
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Permanent (synchronous) hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | No information |
| Generation time | 1-2 years |
| Age at maturity | 1-2 years |
| Season | August - September |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Not relevant |
| Duration of larval stage | No information |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
Age at maturity. In Ireland, Pelvetia canaliculata is at least two years old before it reaches maturity (D. Stengel pers. comm.)
Reproduction. Pelvetia canaliculata produces gametes within receptacles on the tips of the fronds. Receptacles are initiated in January when they start to swell and become distinguishable. In July, the receptacles start ripening and gametes are released from August to early September, after which the receptacles are shed. Gametes are fertilized externally forming zygotes which then settle. A sporeling of 800 µm in length develops in six months. Plants first produce receptacles at a size of 4 to 5 cm long when they are in their first year.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidencePelvetia canaliculata is permanently attached to the substratum, so would be removed upon substratum loss. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | High | Moderate | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceThe effects of smothering depend on the state of the tide when the incident occurred. If smothering took place when the plant was emersed the whole of the plant may be buried under the sediment preventing photosynthesis. If smothering happened while the plant was immersed some of the fronds may escape smothering and be able to continue photosynthesis. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | High | Moderate | Moderate | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceSilt may settle out on the fronds reducing the light available for photosynthesis and therefore lowering growth rates. Siltation may prevent or certainly reduce recruitment (D. Stengel pers. comm.). Once conditions have returned to normal the growth rate would quickly return to normal. | Low | Immediate | Not sensitive | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidencePelvetia canaliculata is very tolerant of desiccation, it can survive emerged for several days after reduction of its water content to just a few percent (Schonbeck & Norton, 1978). However, it cannot withstand desiccation beyond this and an increase in the normal desiccation levels would result in the death of some plants at the uppermost limit of its range. Thus, the upper limit of the Pelvetia canaliculata population would be depressed and so intolerance has been assessed as intermediate. Decreases in the level of desiccation would result in the species being competitively displaced by faster growing species and Pelvetia canaliculata may colonize further up the shore. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | Intermediate | Moderate | Moderate | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceEven on a regular basis, Pelvetia canaliculata can tolerate emersion for up to 8 days. However, those individuals living at the highest level on the shore are living at the top of their physiological tolerance limits and so would not be likely to tolerate an increase in emersion levels. This would result in the upper extent of the species being depressed. Decreases in emersion would result in the species being competitively displaced by faster growing species and Pelvetia canaliculata may colonize further up the shore. The species requires exposure to air to survive, it decays if transplanted further down the shore (Lobban & Harrison, 1997). Thus, because some individuals in the population are likely to be killed intolerance is assessed as intermediate. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | Intermediate | Moderate | Moderate | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAn increase in water flow rate could cause plants to be torn off the substratum or the substratum with the plants attached may be mobilised. Recruitment may be reduced with an increased in water flow rates. A decrease may result in increased siltation which may also reduce recruitment. Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | Intermediate | Moderate | Moderate | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidencePelvetia canaliculata is found in much warmer and much cooler waters than the UK as it is distributed from northern Norway to Spain. It is likely to tolerate a change of 5°C in the short term although it showed some signs of damage during the unusually hot summer of 1983, when the average temperature was 8.3°C higher than normal (Hawkins & Hartnoll, 1985). The effect of such an increase The species is likely to be especially tolerant of a long-term change in temperature of 2°C. | Low | High | Low | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceAn increase in turbidity levels would reduce light levels available for photosynthesis during immersion. However, it has been estimated that Pelvetia canaliculata spends up to 90 percent of its time out of the water (Fish & Fish, 1996) where photosynthesis can still take place whilst the thalli remains wet. Since the algae is able to photosynthesize during the early stages of emersion when the plant is still wet the overall effect of increased turbidity is likely to be minimal. A decrease intolerance has therefore, been recorded as low. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceAn increase in wave exposure may cause Pelvetia canaliculata to be torn off the substratum or the substratum with plants attached may be mobilised. It is unlikely that any recruitment will occur in areas of high wave exposure. In such sites Pelvetia canaliculata can only grow in crevices. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | High | Moderate | Moderate | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceSeaweeds have no known mechanism for perception of noise. | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceSeaweeds have no known mechanism for visual perception. | Tolerant | Not relevant | Not sensitive | Not relevant |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion may kill germlings and damage the fronds of Pelvetia canaliculata. Fucoids are intolerant of abrasion from human trampling which has been shown to reduce fucoid cover on a shore (Fletcher & Frid, 1996; Holt et al., 1997). Therefore, an intolerance of intermediate has been recorded. Recovery rates may be variable. Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event (Keith Hiscock, pers comm.). Therefore, a recoverability of moderate has been recorded. | Intermediate | Moderate | Moderate | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidencePelvetia canaliculata is permanently attached to the substratum so once removed it cannot re-form an attachment. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | High | Moderate | Moderate | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceFucoids are generally robust in the face of chemical pollution (Holt et al., 1997). However, a 50% reduction in growth took place at copper concentrations of 60-80 micrograms per litre. Stromgren (1977) found the growth of Pelvetia canaliculata is actually enhanced by cadmium at concentrations as high as 1000 micrograms per litre although these experiments were not done under natural conditions and may not be applicable in the field (D. Stengel pers. comm.). The reproductive stages are likely to be the most intolerant. | Low | High | Low | Low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidencePelvetia canaliculata disappeared from heavily oiled shores a couple of months after the Amoco Cadiz oil spill (Floc'h & Diouris, 1980). The intolerance of this species is thought to be due to the long residence time of oil on the algae caused by its position high on the shore. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | High | Moderate | Moderate | Low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidencePelvetia canaliculata is adapted to living at low nutrient levels because it can only obtain nutrients when immersed, which may be for as little as 10 percent of its time. A decrease in nutrient levels would lower growth rate in the species. An increase in nutrient levels may cause the species to be overgrown by green algae. However, this is unlikely to have a great effect because most of these species, such as Ulva, are strongly seasonal and opportunistic and have only short life-spans. Recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | Intermediate | Moderate | Moderate | Low |
Increase in salinity [Show more]Increase in salinity
EvidencePelvetia canaliculata must be able to withstand wide variations in salinity because it is usually emerged for long periods of time, during which it will be drenched in freshwater from rain. There is recent evidence (D. Stengel pers. comm.) that although Pelvetia canaliculata can tolerate short-term changes in salinity the physiological balance between the alga and the fungus Mycosphaerella may be disturbed in low-salinity conditions in the long-term. | Low | Very high | Very Low | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceLow oxygen levels are unlikely to affect Pelvetia canaliculata as seaweeds are photoautotrophic and do not need any oxygen for photosynthesis. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidencePelvetia canaliculata can recover quickly from harvesting if plants only greater than 14cm are removed. Otherwise recovery rates may be variable, Subrahmanyan (1960) observed that the species readily recruits to cleared areas of the shore and full recovery of the community takes place within five years. However, in the Shetlands Pelvetia canaliculata did not recolonize shores that had been bulldozed until 7-8 years after the event. | Intermediate | Moderate | Moderate | Moderate |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNR | Not relevant | Not relevant | Not relevant | Not relevant |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Pelvetia canaliculata was historically harvested for use as animal fodder. Sheep still graze Pelvetia canaliculata on the shore in Shetland at least.Bibliography
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Floc'h, J. H. & Diouris, M., 1980. Initial effects of Amoco Cadiz oil on intertidal algae. Ambio, 9, 284-286.
Hardy, F.G. & Guiry, M.D., 2003. A check-list and atlas of the seaweeds of Britain and Ireland. London: British Phycological Society
Hawkins, S.J. & Hartnoll, R.G., 1985. Factors determining the upper limits of intertidal canopy-forming algae. Marine Ecology Progress Series, 20, 265-271.
Holt, T.J., Hartnoll, R.G. & Hawkins, S.J., 1997. The sensitivity and vulnerability to man-induced change of selected communities: intertidal brown algal shrubs, Zostera beds and Sabellaria spinulosa reefs. English Nature, Peterborough, English Nature Research Report No. 234.
Schonbeck, M.W. & Norton, T.A., 1978. Factors controlling the upper limits of fucoid algae on the shore. Journal of Experimental Marine Biology and Ecology, 31, 303-313.
Subrahmanyan, R., 1960. Ecological studies on the Fucales. Part 1. Pelvetia canaliculata Dene. et Thur. The Journal of the Indian Botanical Society, 39, 614 - 630.
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Bristol Regional Environmental Records Centre, 2017. BRERC species records within last 15 years. Occurrence dataset: https://doi.org/10.15468/vntgox accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset: https://doi.org/10.15468/aru16v accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-22
Outer Hebrides Biological Recording, 2018. Non-vascular Plants, Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/goidos accessed via GBIF.org on 2018-10-01.
Royal Botanic Garden Edinburgh, 2018. Royal Botanic Garden Edinburgh Herbarium (E). Occurrence dataset: https://doi.org/10.15468/ypoair accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Algae and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/55albd accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
The Wildlife Information Centre, 2018. TWIC Biodiversity Field Trip Data (1995-present). Occurrence dataset: https://doi.org/10.15468/ljc0ke accessed via GBIF.org on 2018-10-02.
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 29/05/2008