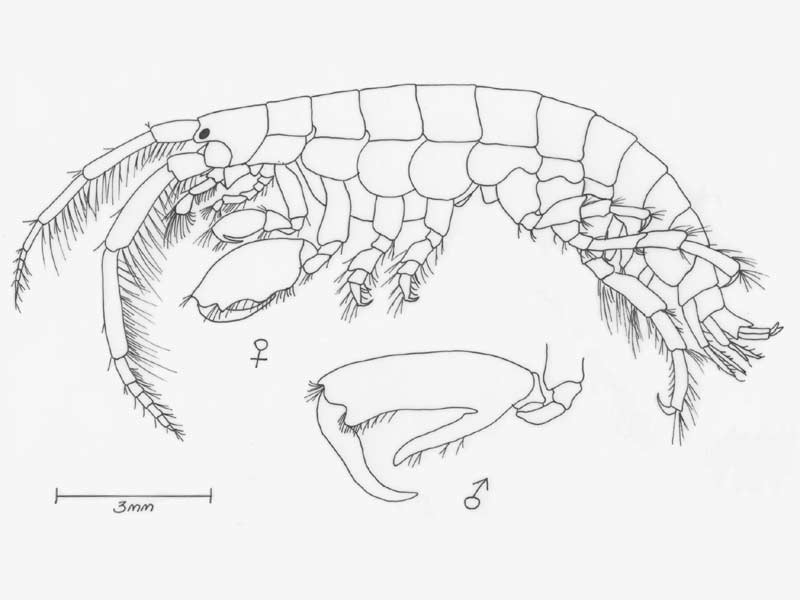
Mottled tube-maker (Jassa falcata)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Jacqueline Hill | Refereed by | Prof. P. Geoff Moore |
| Authority | (Montagu, 1808) | ||
| Other common names | - | Synonyms | - |
Summary
Description
A tube dwelling amphipod, elongate and rather flattened up to 13 mm in length. Colour varied, often yellow-grey with brown, red or black patches depending the colour of the surrounding habitat. Eyes small, round and dark.
Recorded distribution in Britain and Ireland
Found on all British coasts. Reported from several sites around the coast of Ireland.
Global distribution
Cosmopolitan in temperate and warm-temperate waters; widespread and frequently recorded in Atlantic, Pacific and Indian Oceans, both north and south of the equator. Reported from most coastal regions of North East Atlantic, and often locally abundant.
Habitat
Jassa falcata constructs tubes from debris amongst algae, hydroid growths and on solid surfaces in sediment and areas of strong water currents such as pilings, buoys, rafts or the hulls of ships. It is an important fouling organism because the tubes of Jassa falcata often form dense mats or 'nests'. Also found in Laminaria spp. holdfasts and similar habitats.
Depth range
See additional information.Identifying features
- Antennae 1 bears a two segmented accessory flagella and is shorter and more slender than antennae 2.
- Gnathopod 2 larger than gnathopod 1, terminating in characteristic claw which differs in size and form between sexes.
- In adult males, gnathopod 2 propodus is greatly enlarged, with a stout thumb opposing the dactylus.
- Uropod 3 biramous, outer ramus with hooked spine and 1-3 small teeth.
- Telson small, triangular, with two setae on each side of apex.
Additional information
- Jassa falcata shows marked variation in shape and relative proportions of the taxonomically useful characteristics vary with growth stage.
- Therefore, the taxonomy of Jassa falcata has proved to be problematic.
- The species Jassa marmorata and Jassa herdmani are easily confused with Jassa falcata so that some of the literature on Jassa falcata may refer to these species or a mixture of Jassa species (Conlan, 1990).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Family | Ischyroceridae | |
| Genus | Jassa | |
| Authority | (Montagu, 1808) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 7-13 mm | ||
| Male size at maturity | 3.11-11.6 mm | ||
| Female size range | 3.8-6.6 mm | ||
| Female size at maturity | |||
| Growth form | Articulate | ||
| Growth rate | 0.07mm/day | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Swimmer (appendages, paddles) | ||
| Characteristic feeding method | Active suspension feeder, Predator, See additional information | ||
| Diet/food source | See additional information | ||
| Typically feeds on | Unselective suspension feeder of detritus and small organisms. | ||
| Sociability | |||
| Environmental position | Epibenthic | ||
| Dependency | No information found. | ||
| Supports | No information | ||
| Is the species harmful? | No | ||
Biology information
- Jassa falcata builds tubes, from debris, among algae and hydroids, and on solid surfaces and structures. Males and females live in separate tubes constructed of pieces of debris cemented together, the tubes often forming dense mats.
- This species is essentially a benthic tube dwelling amphipod although intermittent swimming and crawling is common.
- Jassa falcata is generally a suspension feeder, however, large specimens have also been seen to capture and feed upon other smaller amphipods and ostracods to supplement their diet.
- Growth rate is given as maximum growth rate observed in laboratory investigations at varying temperatures. Maximum growth took place at 20 °C (Nair & Anger, 1979).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Open coast, Strait or Sound |
| Biological zone preferences | Lower eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Macroalgae, Other species |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed, Sheltered, Very exposed |
| Salinity preferences | Full (30-40 psu) |
| Depth range | See additional information. |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
This species is an important fouling organism. The animals construct tubes from pieces of debris and sediment cemented together, often forming dense mats, particularly in warm water discharge pipes from power stations (Fish & Fish, 1996). Reported to depths of five metres in Japan (Kamenskaya, 1977). Jassa falcata is often found inhabiting Laminaria holdfasts and so may be found at the greater depths inhabited by some kelps. Jassa falcata appears to be out-competed by Parajassa pelagica or unable to survive in very strong wave action at shallow depths (K. Hiscock personal communication).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | 11-100 |
| Generation time | <1 year |
| Age at maturity | 2-6 months |
| Season | See additional text |
| Life span | <1 year |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Direct development |
| Duration of larval stage | Not relevant |
| Larval dispersal potential | No information |
| Larval settlement period | Not relevant |
Life history information
- Reproduction, and therefore production of gametes, occurs throughout the year although some seasonal peaks of reproduction have been observed. In Helgoland waters, for example, two peaks were observed, the main one in summer and another smaller peak in winter (Nair & Anger, 1980).
- The mating system is polygynous. Several broods of offspring are produced, each potentially fertilised by a different male. Males are believed to seek out mature females attracted by female pheromones.
- There is no sperm storage, and fertilisation is external.
- There is no larval stage. Embryos are brooded in a marsupium, beneath the thorax, formed by the oostegites (a series of flattened plates projecting from basal segments). Embryos are released as subjuveniles with incompletely developed eighth thoracopods and certain differences in body proportions and pigmentation.
- In laboratory investigations, lifespan, time to maturity and fecundity were strongly influenced by temperature (Nair & Anger, 1979). At 20 °C the time to reach maturity is 2 months, about half the value observed at 10 °C. Field investigations in Helgoland observed age at maturity to be 6 months for new generations produced in low winter temperatures of 7-8 °C (Nair & Anger, 1980).
- Growth rate also increases with increasing temperature but lifespan is shorter and individuals are smaller at higher temperatures (Nair & Anger, 1979).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceSubstratum loss will remove the tubes in which Jassa falcata lives. Although the species is mobile it is a weak swimmer and so likely that only some individuals would escape and rebuild tubes elsewhere. However, Jassa falcata does not have a larval stage so dispersal potential is limited (Hughes, 1979) although rafting on algal debris is probably important in extending the species range (Moore, P.G., pers. comm.). In a New England sub-tidal zone for example, Jassa falcata recolonized areas of cleared rock within 4 months (Sebens, 1986). | Intermediate | Moderate | Moderate | Very low |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering of the population by sediment to a depth of 5cm above the substratum may completely cover Jassa falcata tubes and prevent suspension feeding. The species is likely to be able to crawl away from the sediment but as a poor swimmer and with the absence of a larval stage it has limited dispersal potential (Hughes, 1979). However, recolonization from adjacent populations can take place in less than a year and rafting on algal debris is probably important in extending the species range (Moore, P.G., pers. comm.). In a New England sub-tidal zone for example, Jassa falcata recolonized areas of cleared rock within 4 months (Sebens, 1986). | Intermediate | Moderate | Moderate | Very low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceJassa falcata is tolerant of high turbidity (Moore, 1973(b)) and uses debris in the construction of its tube and so is likely to be tolerant of siltation at the level of the benchmark, 100mg/l for a month. In investigations in France all Jassa falcata tubes on hydroids and algae were open at both ends (Conlan, 1989) and Moore (1977) speculates that this may be adaptive in avoiding siltation. | Low | High | Low | Very low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceBousfield (1973) suggests the tolerance of amphipods to desiccation is generally quite low. Jassa falcata has been observed fully exposed to air at low tide, though normally remaining within its tube (Conlan, 1989). Jassa falcata is a sub-tidal and lower shore species so an increase in desiccation at the level of the benchmark is likely to reduce the extent of the population. | Intermediate | High | Low | Very low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceJassa falcata has been observed amongst red algae fully exposed to the air and so can tolerate some emergence. Some individuals were crawling amongst the tubes, but most remained within (Conlan, 1989). Increased emergence may decrease the upper limit of Jassa falcata populations, whereas decreases in emergence may allow the species to colonize further up the shore. | Low | High | Low | Very low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceJassa falcata is morphologically adapted to rough hydrodynamic environments since it is able to remain within its tube and its stout gnathopods can hold tightly to algae. Ebling et al. (1948) investigated the fauna of the Saccorhiza polyschides canopy at Lough Ine, Ireland with reference to water currents. Jassa falcata was abundant at all stations where current was strong (4-6 knots), occurred in small quantities in moderate current (2-3 knots) and was absent from all stations where current was weak (1 knot). Weak currents interfere with feeding and Jassa falcata is sometimes outcompeted in weak currents by another tube dwelling amphipod, Corophium insidiosum that is able to switch from suspension feeding to deposit feeding in low flow water rates. (Nair & Anger, 1980). | Low | High | Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceJassa falcata occurs in temperatures from 1.5-27 °C in the Sea of Japan (Kamenskaya, 1977). Bousfield (1973), however, reports that amphipod tolerance to extremes of temperature is low. Life span, growth rates, size at maturity and fecundity are all affected by temperature. In Helgoland, hardly any individual growth was observed at temperatures of 2.5-4.4 °C and growth was rapid at maximal temperatures of about 17°C. Peak reproduction occurred at temperatures of 10-14 °C and egg numbers per gravid females were higher, 92-100 in summer months compared to about 10 eggs in winter (Nair & Anger, 1980). In laboratory investigation Jassa falcata showed a slow, long-continued growth at 10°C resulting in a prolonged life span and a larger final body size (Nair & Anger, 1979). Although growth and reproduction are influenced by temperature no information on upper or lower lethal temperature limit was found. | Intermediate | Moderate | Moderate | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceIn a study of kelp holdfast fauna in north east Britain, Moore (1973(b)) found Jassa falcata a ubiquitous species but with higher abundances in turbid waters. This species was also found to favour waters with high turbidity in Los Angeles - Long Beach harbours (Barnard, 1958) and was absent from clear waters. Mills (1967) suggested that turbidity of the water might be responsible for initiating feeding in some tube-dwelling amphipods. | Low | High | Low | Very low |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceAlthough found on most coasts around the UK, the density of Jassa falcata increases with greater wave exposure (Moore, 1985). With stout gnathopods Jassa falcata is adapted for rough hydrodynamic environments. | Low | High | Low | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceJassa falcata often inhabits areas such as buoys and pilings in harbours where noise and vibration levels are likely to be high. It is likely therefore, that the species is tolerant of noise. | Tolerant | Not relevant | Not sensitive | Very low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceJassa falcata often inhabits areas such as buoys and pilings in harbours where visual disturbance at the level of the benchmark, continuous presence for one month of moving objects such as boats, commonly occurs and its visual acuity is probably low. It is likely therefore, that the species is not sensitive to visual disturbance. | Tolerant | Not relevant | Not sensitive | Very low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion at the level of the benchmark would be likely to kill or displace some individuals. However, since Jassa falcata lives in colonies the whole population may not be destroyed and so intolerance is assessed as intermediate. Recovery is good because recolonization from adjacent populations can take place within 4 months (Sebens, 1986). | Intermediate | High | Low | Very low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceDisplacement may result in the death of loss of individuals, however if physically removed from their original position onto a suitable substratum Jassa falcata would be able to construct a new tube. In the absence of a larval stage dispersal of Jassa falcata normally occurs by short distance dispersal by juveniles so recolonization should be rapid. In a New England sublittoral community, for example, Jassa falcata recolonized areas of cleared rock within 4 months (Sebens, 1986). However, after the last moult silk spinning glands in males are reduced in size limiting the tube building ability of sexually mature adult males. | Low | High | Low | Very low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. Evidenceintolerance to some chemicals has been observed in amphipods. Gammaridean amphipods have been reported to be intolerant of TBT with 10 day LC50 (the concentration which produces 50% mortality) values of 1-48ng/l (Meador et al., 1993). Jassa falcata was absent from sites close to a bromide extraction plant releasing acidified halogenated effluent (Hoare & Hiscock, 1974). | Intermediate | Moderate | Moderate | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceCrustaceans are generally regarded to be sensitive to cadmium (McLusky et al., 1986). In laboratory investigations Hong & Reish (1987) observed 96hour LC50 (the concentration which produces 50% mortality) water column concentrations of between 0.19 and 1.83 mg/l for several species of amphipod. | Intermediate | Moderate | Moderate | Very low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceAmphipods in general are highly sensitive to oil pollution (Suchanek, 1983). After the Amoco Cadiz oil spill, for example, there was a reduction in both the number of amphipod species and the number of individuals (Cabioch et al., 1978). Eight years after the oil spill amphipod numbers had still not fully recovered (Dauvin, 1987). Analysis of kelp holfast fauna after the Sea Empress oil spill in Milford Haven showed an almost complete lack of any amphipods at the badly oiled sites, while large numbers of Jassa falcata were present at unaffected sites (SEEEC, 1998). | High | Very High | High | |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation. | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceIn a study of amphipods in north east Britain Jassa falcata was found to prefer turbid waters, in particular those unpolluted by industrial and domestic waste including sewage (Moore, 1973(b)). However, in other studies of sites polluted with industrial and sewage waste Jassa falcata was more abundant (Bellan-Santini, 1980). Information about the exact nature of the pollution is not available and so an assessment of intolerance to nutrients is not possible. | No information | No information | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceAt moorings, buoys and piers in the Sea of Japan Jassa falcata was the dominant species at salinities of 30-33psu but at lower salinities Corophiidae were more abundant (Kamenskaya, 1977). Therefore, at lower salinities there may be increased risk of competition from other tube-building amphipods. | No information | No information | No information | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceIn a survey of Los Angeles to Long Beach harbours Jassa falcata was absent from areas of low oxygen concentration (0-2.5mg/l). However, recovery was rapid with recolonization taking place within 6-9 months (Barnard, 1958). | High | High | Moderate | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficientinformation. | No information | No information | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceInsufficientinformation. | No information | No information | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceRecovery from extraction of 50% of individuals is likely to be good because Jassa falcata reproduces all year round. Recolonization from existing individuals in the same area can take place within less than one year (Barnard, 1958). In New England, Jassa falcata had recolonized areas of cleared subtidal rock within 1-4 months (Sebens, 1986). | Intermediate | High | Low | Very low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceLaminaria spp. used by the amphipod as substratum may be harvested for commercial use. Epiphytic or holdfast populations may be lost or displaced. However, a protracted reproductive period means that successful recruitment and recolonization from adjacent populations is likely. In New England, for example, Jassa falcata recolonized areas of cleared rock within 4 months (Sebens, 1986). | Intermediate | High | Low | Very low |
Additional information
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | Native |
| Origin | - |
| Date Arrived | - |
Importance information
Jassa falcata populations can form dense tube mats, particularly on solid surfaces such as harbour structures and ships hulls, that may exclude other species. Tube mats may provide refugia for other small invertebrates.The colonies or 'nests' of Jassa falcata become very large and can cause congestion of water pipes and ducts which run into the sea from power stations or similar installations (Lincoln, 1979).
Bibliography
Baardseth, E., 1970. Synopsis of the biological data on knotted wrack Ascophyllum nodosum (L.) Le Jolis. FAO Fisheries Synopsis, no. 38, Rev. 1.
Barnard, J.L., 1958. Amphipod crustaceans as fouling organisms in Los Angeles-Long Beach harbours, with reference to the influence of seawater turbidity. California Fish and Game, 44, 161-170.
Barnes, R.D., 1980. Invertebrate Zoology, 4th ed. Philadelphia: Holt-Saunders International Editions.
Bellan-Santini, D., 1980. Relationship between populations of amphipods and pollution. Marine Pollution Bulletin, 11, 224-227. https://doi.org/10.1016/0025-326X(80)90411-7
Bieler, R., 1992. Gastropod phylogeny and systematics. Annual Review of Ecology and Systematics, 23, 311 -338.
Bousfield, E.L., 1973. Shallow-water gammaridean Amphipoda of New England. London: Cornell University Press.
Cabioch, L., Dauvin, J.C. & Gentil, F., 1978. Preliminary observations on pollution of the sea bed and disturbance of sub-littoral communities in northern Brittany by oil from the Amoco Cadiz. Marine Pollution Bulletin, 9, 303-307.
Conlan, K.E., 1989. Delayed reproduction and adult dimorphism in males of the amphipod genus Jassa (Corophioidea: Ischyroceridae): an explanation for systematic confusion. Journal of Crustacean Biology, 9, 601-625.
Conlan, K.E., 1990. Revision of the crustacean amphipod genus Jassa Leach (Corophioidea: Ischyroceridae). Canadian Journal of Zoology, 68, 2031-2075.
Costello, M.J., Holmes, J.M.C., McGrath, D. & Myers, A.A., 1989. A review and catalogue of the Amphipoda (Crustacea) in Ireland. Irish Fisheries Investigations. Series B (Marine), (33), 70pp.
Dauvin, J.C., 1987. Evolution a long terme (1978-1986) des populations d'amphipodes des sables fins de la Pierre Noire (Baie de Morlaix, Manche Occidentale) apres la catastophe de l'Amoco Cadiz. Marine Environmental Research, 21, 247-273.
Ebling, F.J., Kitching, J.A., Purchon, R.D. & Bassingdale, R., 1948. The ecology of Lough Ine rapids with special reference to water currents. 2. The fauna of the Saccorhiza canopy. Journal of Animal Ecology, 17, 223-244.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Hong, J. & Reish, D.J., 1987. Acute toxicity of cadmium to eight species of marine amphipod and isopod crustaceans from southern California. Bulletin of Environmental Contamination and Toxicology, 39, 884-888.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Hughes, R.G., 1979. The dispersal and dispersion of some epizoites of the hydroid Nemertesia antennina (L.) Journal of the Marine Biological Association of the United Kingdom, 59, 879-887.
Kamenskaya, O.E., 1977. Amphipods in the fouling of hydrotechnical installations in the sea of Japan. Soviet Journal of Marine Biology, 3, 375-379.
Lincoln, R.J., 1979. British Marine Amphipoda: Gammaridea. London: British Museum (Natural History).
McLusky, D.S., Bryant, V. & Campbell, R., 1986. The effects of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanography and Marine Biology: an Annual Review, 24, 481-520.
Meador, J.P., Varanasi, U. & Krone, C.A., 1993. Differential sensitivity of marine infaunal amphipods to tributyltin. Marine Biology, 116, 231-239.
Moore, P.G., 1973b. The kelp fauna of north east Britain. II. Multivariate classification: turbidity as an ecological factor. Journal of Experimental Marine Biology and Ecology, 13, 127-163.
Moore, P.G., 1977a. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanography and Marine Biology: An Annual Review, 15, 225-363.
Moore, P.G., 1985. Levels of heterogeneity and the amphipod fauna of kelp holdfasts. In The Ecology of Rocky Coasts: essays presented to J.R. Lewis, D.Sc. (ed. P.G. Moore & R. Seed), 274-289. London: Hodder & Stoughton Ltd.
Nair, K.K.C. & Anger, K., 1979. Experimental studies on the life cycle of Jassa falcata (Crustacea, Amphipoda). Helgolander Wissenschaftliche Meeresuntersuchungen, 32, 444-452.
Nair, K.K.C. & Anger, K., 1980. Seasonal variation in population structure and biochemical composition of Jassa falcata (Crustacea, Amphipoda) off the island of Helgoland (North Sea). Estuarine and Coastal Marine Science, 11, 505-513.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Sebens, K.P., 1986. Spatial relationships among encrusting marine organisms in the New England subtidal zone. Ecological Monographs, 56, 73-96. DOI https://doi.org/10.2307/2937271
SEEEC (Sea Empress Environmental Evaluation Committee), 1998. The environmental impact of the Sea Empress oil spill. Final Report of the Sea Empress Environmental Evaluation Committee, 135 pp., London: HMSO.
Suchanek, T.H., 1993. Oil impacts on marine invertebrate populations and communities. American Zoologist, 33, 510-523. DOI https://doi.org/10.1093/icb/33.6.510
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-22
South East Wales Biodiversity Records Centre, 2018. SEWBReC Myriapods, Isopods, and allied species (South East Wales). Occurrence dataset: https://doi.org/10.15468/rvxsqs accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 08/08/2000