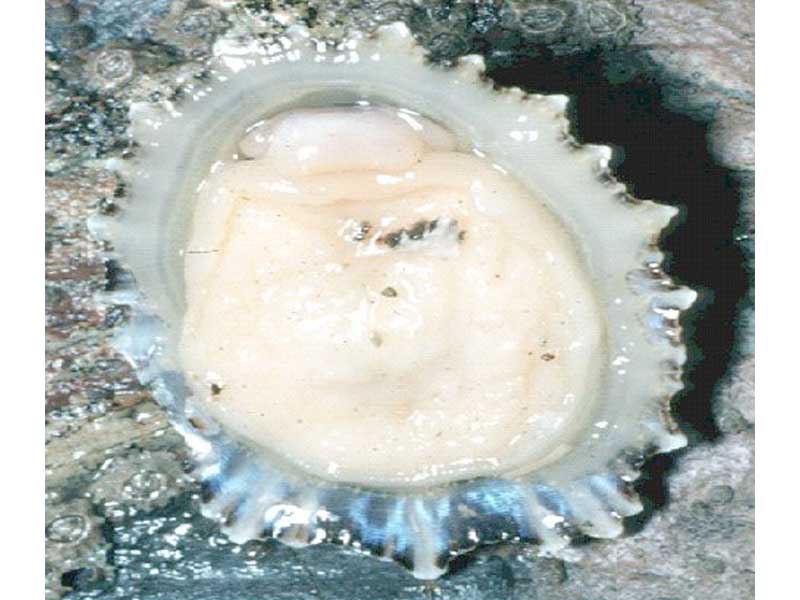
China limpet (Patella ulyssiponensis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Ken Neal & Marie Skewes | Refereed by | This information is not refereed |
| Authority | Gmelin, 1791 | ||
| Other common names | - | Synonyms | Patella aspera Röding, 1798 |
Summary
Description
Shell forming a low cone with ridges on the outer surface that project noticeably around the edges of the shell. Patella ulyssiponensis reaches up to 6 cm in length and the apex is noticeably anterior to the centre. The outer surface of the shell is a whitish-grey while the inner surface is a porcellanous white, with a yellow or orange hint towards the apex. The sole of the foot is orange or yellow, and the mantle is edged with translucent white tentacles.
Recorded distribution in Britain and Ireland
The china limpet is found around most of the coast of the British Isles, reaching its northern limit in the Shetland Isles. Absent or rare on south-east shores of England from the Humber Estuary to the Isle of Wight.Global distribution
Patella ulyssiponensis is a southern (Lusitanian) species extending south to the Mediterranean.Habitat
Common on exposed rocky shores, avoiding extreme shelter and low salinities. Present on the lower shore and, rarely, in the shallow sublittoral. Also occurring in shallow rock pools on the middle shore and on overhanging rocks and the sides of gullies.Depth range
IntertidalIdentifying features
- Radiating ridges on the outer surface, often with tubercles where crossed by growth lines and projecting noticeably around the edges of the shell.
- Characteristic pattern of alternating single and triple ridges.
- Anterior end of shell distinctly narrower than posterior.
- The sole of the foot is orange or yellow and the mantle is edged with translucent white tentacles.
Additional information
Patella ulyssiponensis was formerly known as Patella aspera.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Gastropoda | Snails, slugs & sea butterflies |
| Family | Patellidae | |
| Genus | Patella | |
| Authority | Gmelin, 1791 | |
| Recent Synonyms | Patella aspera Röding, 1798 | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 0.25 - 58mm | ||
| Male size at maturity | 20mm | ||
| Female size range | 18mm | ||
| Female size at maturity | |||
| Growth form | |||
| Growth rate | 5 - 7mm/year | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | |||
| Characteristic feeding method | |||
| Diet/food source | |||
| Typically feeds on | Epilithic algae and biofilms. | ||
| Sociability | |||
| Environmental position | Epibenthic | ||
| Dependency | No text entered. | ||
| Supports | See additional information | ||
| Is the species harmful? | No | ||
Biology information
On rocky shores of wave exposure grade 1 (Ballantine scale: Ballantine, 1964), Patella ulyssiponensis occurs at densities of 1000 m² but individuals are small (Thompson, 1979). On less wave exposed shores density is lower but individuals are larger.
Patella ulyssiponensis is parasitised by Cercaria patellae, a trematode platyhelminth, which have infection levels of 5-10% in adults and can cause damage of the digestive gland. Gymnophallid metacercariae infect between the mantle and the shell and have an infection level of approximately 5%. The gut of Patella ulyssiponensis is sometimes infected by larval cyclophyllidean tapeworms (Kinne, 1980).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast |
| Biological zone preferences | Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Bedrock |
| Tidal strength preferences | Strong 3 to 6 knots (1.5-3 m/sec.), Very strong > 6 knots (>3 m/sec.) |
| Wave exposure preferences | Exposed, Extremely exposed, Very exposed |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | Intertidal |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
In wave exposed situations, Patella ulyssiponensis is the commonest limpet on the lower shore.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Protandrous hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | No information |
| Generation time | 2-5 years |
| Age at maturity | 3 years |
| Season | August - October |
| Life span | 11-20 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 2-10 days |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
Maturation of gonads begins in May/June and all mature individuals have ripe gonads by mid-August. Spawning occurs in October and is believed to be triggered by strong gales (Thompson, 1979). The sex ratio of this species varies with size of individual. For example, at 20 mm shell length, all mature individuals are male, while from 20 mm to full size the number of females increases until at 55 mm around 70% of the mature individuals are female (Thompson, 1979).Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceThe species is epifaunal so loss of the substratum would also result in loss of the population. Individuals unattached to the substratum are very vulnerable to desiccation and to predation by birds and crabs. An intolerance of high has therefore been recorded. For recoverability, see additional information below. | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceA related limpet, Patella granularis, was found to survive no more than 3 days when smothered with sand. This effect was thought to be due to hypoxia caused by the sand preventing a normal current of water passing over the gills (Marshall & McQuaid, 1989). Also, inundation with sand is likely to affect locomotion and grazing and cause the limpet to starve (Professor Steve Hawkins, pers. comm.). Therefore an intolerance of high has been recorded. For recoverability, see additional information below. | High | High | Moderate | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceSince Patella ulyssiponensis is a grazer on rocky shores, an increase in suspended sediment is unlikely to reduce its ability to find food. Predation rates are unlikely to be affected since its principal underwater predators (crabs and starfish) use senses other than sight to locate prey and birds (such as oystercatchers) prey on limpets when they are exposed by the tide. An increase in suspended sediment may clog the gills of limpets and lead to difficulties in extracting oxygen from the water. Intolerance is assessed as low. | Low | Very high | Very Low | Very low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidencePatella ulyssiponensis is not reliant on suspended sediment for food or shelter so a decrease is unlikely to affect this species. Therefore tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | |
Desiccation [Show more]Desiccation
EvidencePatella ulyssiponensis suffers 100% mortality at 40% water loss. In comparison, Patella vulgata suffers at most 30% mortality at the same water loss (Davies, 1969). Therefore, at the benchmark level, an intolerance of high has been recorded. | High | High | Moderate | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidencePatella ulyssiponensis is found on the lower shore and in rock pools in the mid-shore. If emergence increased adults may migrate down the shore although it is also possible that some individuals would stick to their home scars and die. Therefore an intolerance of intermediate has been recorded reflecting some mortality. | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceSome Patella ulyssiponensis occur subtidally, therefore an decrease in emergence would probably not affect this species. Therefore tolerant has been recorded. | Tolerant | Immediate | Not sensitive | Low |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceLimpets are extremely tenacious and tests on one species, Lottia pelta, showed that they will only begin to be dislodged by water currents in excess of 13 m/s (25 knots) while stationary and 8 m/s (16 knots) while moving. To remove all the limpets from a surface would take current speeds in excess of 20 m/s (39 knots) (Denny, 1989). Tidal currents in the waters of Britain and Ireland do not exceed 10 knots in surface velocity. Therefore tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceIf the reduction in water flow rate was concomitant with a reduction in wave exposure then Patella ulyssiponensis may become less abundant or disappear entirely. Under any other circumstances, a decrease in water flow rate is unlikely to affect this species. Therefore tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceGrazing activity in limpets is closely correlated with temperature: the warmer it is, the more time limpets spend grazing (Jenkins et al., 2001). A slight warming is likely to be beneficial to individual and population growth. Patella ulyssiponensis is less tolerant than Patella vulgata to changes in temperature. However, since it occurs in the sublittoral fringe and in rock pools in the mid-shore, changes in temperature at the benchmark level are likely to be buffered. Therefore tolerant has been recorded for an increase in temperature. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceA related species, Patella vulgata, loses adhesion at very low temperatures and become very vulnerable to crab and bird predation (see review of Patella vulgata). The water in a rock pool will buffer sudden and drastic changes in air temperature until the tide returns with warmer water and thus protect any organisms within the pools. In the very cold winter of 1962-63 Patella ulyssiponensis suffered less mortality than other limpets because it was protected by the warmer seawater because it was not exposed to the cold air temperatures for very long each day. An intolerance of intermediate has been recorded for a decrease temperature because extreme cold in winter is likely to kill a proportion of the population. | Intermediate | High | Low | Low |
Increase in turbidity [Show more]Increase in turbidity
EvidenceIncreasing turbidity is unlikely to directly affect Patella ulyssiponensis but may reduce the amount of light reaching the epilithic algae at high tide. This will reduce the productivity of the epilithic algae and may affect the growth and reproductive output of the organisms which feed upon it (Langston et al., 2003). Therefore tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceA decrease in turbidity is likely to benefit to all rocky shore grazers by increasing the amount of light reaching the epilithic algae and increasing productivity which will probably increase the productivity of the grazers. Therefore tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceLimpets are extremely tenacious and Patella ulyssiponensis occurs at the highest densities on the most wave exposed shores: up to 1000 m² on extremely exposed shores (Thompson, 1979). Therefore an increase in exposure is unlikely to affect this species. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidencePatella ulyssiponensis prefers wave exposed shores with little fucoid cover and is intolerant to a reduction in wave exposure (Evans, 1953). However the effect of a decrease in wave exposure on a population of Patella ulyssiponensis depends on the wave exposure before the decrease. The benchmark is a 2 rank change in the wave exposure of the shore. Therefore, if the Patella ulyssiponensis population started at extremely exposed and ended up at exposed, little change in the population would occur since fucoids only start to appear on moderately exposed/sheltered shores (Little & Kitching, 1996) and Patella ulyssiponensis is tolerant to sparse fucoid cover (Evans, 1953). However, if the population started on an exposed shore and ended up on a sheltered shore, the Patella ulyssiponensis are likely to become locally extinct in that area until the returns to its previous exposure grade. An intermediate intolerance of a reduction in wave exposure has therefore been recorded to take into account the worst case scenario described above. | Intermediate | High | Low | Moderate |
Noise [Show more]Noise
EvidenceAlthough limpets are not likely to be affected by atmospheric noise levels, vibrations near to the animal will cause the shell muscles to contract vigorously, clamping the limpet to the rock (Fretter & Graham, 1994), and potentially interfere with respiration and foraging in pools. This is unlikely to seriously affect Patella ulyssiponensis at the benchmark level and therefore tolerant has been recorded. | Tolerant | High | Not sensitive | Low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidencePatella ulyssiponensis has eyes and is sensitive to visual presence. The limpet may clamp down onto its home scar in response to, for example, the presence of humans on the shore. However, this reaction is short lived and is unlikely to adversely affect the viability of the species. Therefore tolerant has been recorded. | Tolerant | Very high | Not sensitive | Low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe adult has a tough shell that offers protection from abrading factors and any near vibration causes the shell muscles to contract vigorously, clamping the animal to the rock. A short, sharp knock may dislodge an individual leaving it vulnerable to predation and limpets may be crushed by wave driven debris or by seagoing vessels grounding on the shore. However, small individuals tend to occupy depressions, crevices, or pools that would provide protection from trampling or the scraping that would occur when a vessel goes ashore. Therefore, an intolerance of intermediate has been recorded. | Intermediate | High | Low | Very low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceLimpets are intolerant of being knocked off the rock by trampling on the shore and, if the foot is damaged, do not re-attach easily (Professor Steve Hawkins, pers. comm.). Displaced individuals with the foot exposed to the air are likely to become prone to predation and desiccation and may die. If individuals remain foot down on rock after displacement and are not damaged they may be able to become reattached. However, individuals removed several feet from their scars do not appear to make their way home again (Fretter & Graham, 1996) and so may be more vulnerable to desiccation without the tight fit to their 'home scar'. Therefore, an intolerance of intermediate has been recorded. | Intermediate | High | Low | Very low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceLimpets are extremely intolerant of fresh oil and any solvent based dispersants used in oil spill clean-up. During the clean-up response to the Torrey Canyon oil spill, nearly all the limpets were killed in areas close to dispersant spraying (Southward & Southward, 1978). Viscous oil will not be readily drawn in under the edge of the shell by ciliary currents in the mantle cavity, whereas detergent, alone, or diluted in sea water, would creep in much more readily and be liable to kill the limpet (Smith, 1968). A concentration of 5 ppm of chemical dispersant killed half the limpets tested in 24 hours (Southward & Southward, 1978; Hawkins & Southward, 1992).Sub-lethal effects on limpets are also relevant. The limpets may be narcotized by the fresh oil which may render them incapable of clamping down on their home scar. Consequently they may be more vulnerable to predation since they would be easier to prize off the rocks. In addition, they may be more susceptible to dislodgment by, for example, wave exposure and wave driven debris. When limpets move around on the rock they leave a mucous trail which enables them to retreat back to their home scar. If contact with this mucous trail is broken it is unlikely that the limpet will successfully find its way back to its home scar. This will also leave the limpet more prone to predation.
Acidified sea-water affects the motility of Patella vulgata. At a pH of 5.5, motility was reduced whilst submerged but individuals recovered when returned to normal sea-water. At a pH of 2.5, total inhibition of movement occurred and when returned to normal sea-water half had died (Bonner et al., 1993). Reduced motility reduces time for foraging and may result in decreased survival of individuals. Acidified seawater can also change the shell composition which will lead to a decrease in its protective nature and hence survival (Bonner et al., 1993). Short periods (48 hours) are unlikely to have much effect on a population but long periods (1 year) may cause reduced grazing and an increase in algal growth. However, sea-water is unlikely to reach pH 2.5 therefore intolerance to slight changes in pH will be low. Gastropod molluscs are known to be intolerant of endocrine disruption from synthetic chemicals such as tri-butyl tin (Cole et al., 1999). However no information on the specific effects of tri-butyl tin on Patella vulgata was found. Hoare & Hiscock (1974) reported that in Amlwch Bay Patella vulgata was excluded from sites within 100-150 m of the discharge of acidified, halogenated effluent. Therefore an intolerance of high has been recorded.
| High | High | Moderate | High |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceLimpets are regarded as good bioindicators of heavy metal pollution because they tend to accumulate metals at levels proportional to their environment (Catsiki et al., 1991). Limpets isolate and detoxify heavy metals by producing metallothioneins in response to heavy metal pollution (Bebianno et al., 2003). Metallothioneins are low molecular weight proteins that bind metal cations, their production allows an organism to tolerate heavy metal pollution but presumably have a metabolic cost that increases with greater heavy metal exposure. Limpets also adjust their physiology and behaviour in response to heavy metal exposure. Exposure of Patella ulyssiponensis to 0.5 ppm of copper caused the limpets' hearts to stop beating (probably to prevent metal absorbed through the gills travelling around the body) but heart activity returned to normal once the limpets were returned to clean seawater (De Pirro et al., 2001). Exposure to solutions of copper and zinc were found to suppress activity in Patella ulyssiponensis by causing them to clamp their shell onto the substratum and thus isolate them from the environment (Davies, 1992). In this way, Patella ulyssiponensis can tolerate short pulses of heavy metal input but longer term exposure will have energetic consequences for the limpets. In summary, limpets will be stressed by exposure to heavy metals but widespread mortality is unlikely and therefore their intolerance is recorded as low. | Low | High | Low | High |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceThe resistance of Patella ulyssiponensis to direct oiling is poor and causes high mortalities. Weathered oil that has been deposited on the bedrock of the shore gets scraped off during grazing by limpets and ends up in their digestive system. This does not seem to affect them, presumably because there is little of the lighter, more toxic fractions of the oil left (Southward & Southward, 1978).Petpiroon & Dicks (1982) studied the long term effects an oil refinery discharge had on the surrounding shore communities in Littlewick Bay, Milford Haven. By combining their own field work with earlier studies in the same area it was evident that numbers of Patella species had fallen considerably at the two sample sites nearest the discharge pipe (the pipe being approximately 100 m away) since the 1960s. Individuals that were found within the vicinity of the pipe were comparatively much larger. In addition, no juveniles were found within about 45 m of the outfall suggesting that larval recruitment was affected either through the direct mortality of the larvae or by inhibiting larval settlement on the substratum surrounding the pipe. This would also explain the lack of younger, smaller individuals nearest the pipe. The maximum oil content in the effluent gradually decreased from 50 ppm at the start of the work to 25 ppm at the end of the study. Fieldwork in 1981 (the end of the study) found that some juveniles had settled at the two sampling stations nearest the pipe. However the reduction in oil content was not thought to have corresponded with any reduction in the biological effects of the effluent. Petpiroon & Dicks (1982) suggested that at least part of the observed effects resulted from the effluent effects on the larval stages as opposed to the adults directly. In addition, the low salinity of the effluent and the sheltered nature of the receiving waters had meant that the effluent floated after discharge and as a result made contact with all levels of the shore during each tide. Overall, due to the lethal effect that direct oiling has on the limpets an intolerance of high has been recorded. | High | High | Moderate | High |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information found. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceLimpets near sewage outfalls have a higher growth rate than those further away but mortality is higher. However the source of mortality was unknown (Tablado et al., 1994). In another study, there was a massive mortality of limpets 2-3 weeks after a nearby sewage outfall was shut off permanently: the nutrient enrichment from the outfall had led to a dense growth of sea lettuce, Ulva lactuca, which supported a very large population of limpets. Once the enrichment ceased, the Ulva lactuca could not recover from grazing damage as quickly as before and was eventually completely removed from the area around the outfall. With their food source eradicated, a large proportion of the limpets starved (Rogers, 2003). Limpets often benefit from nutrient enrichment (Rogers, 2003 and Tablado et al., 1994) and can survive extended periods the anoxia (Santini et al., 2001) that excludes many organisms from areas of organic enrichment. A drop in nutrient levels is more difficult to assess. Mass mortalities of limpets have been described when artificial enrichment is removed but this probably represents a return to an environmental equilibrium in place before the enrichment occurred. It can only be assumed that if nutrient decreased in an area with no previous enrichment that the limpets' growth would be slowed and that reproductive output would also be reduced. As nutrient further decreased, the population would presumably thin out as some individuals starved. Based upon the evidence of increasing population size with organic enrichment, an intolerance of tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | High |
Increase in salinity [Show more]Increase in salinity
EvidenceIn laboratory experiments, Patella ulyssiponensis survived 43psu for 24 minutes and was only killed when placed in 63 psu for 24 minutes. The metabolic rate of the limpets increased in increasing salinity and this was thought either to be a result of increased locomotion (an escape response) or maintenance of cell volume (De Pirro et al. 1999). Gastropods are osmoconformers, meaning that the salinity of their body fluids is equal to that of their surroundings but in high salinities, water will leave their cells so metabolism has to increase to pump water back into cells to maintain their volume. Because of the various ways Patella ulyssiponensis can cope with an increase in salinity, an intolerance of low has been recorded. | Low | High | Low | High |
Decrease in salinity [Show more]Decrease in salinity
EvidencePatella ulyssiponensis does not penetrate into estuaries although its tolerance of short periods of low salinities is high (Evans, 1953). Lack of penetration into estuaries may be because of increased shelter. Rainfall can significantly lower the salinity of the rock pools it inhabits. Patella ulyssiponensis survived 24 minute exposures at 23 psu without apparent physiological effect but there was 100% mortality when exposed to 3 psu for more than 20 minutes. The limpets' response to low salinity was the same as when exposed to high salinity i.e. metabolic rate increased (De Pirro et al., 1999). It seems that Patella ulyssiponensis occurs only in full and variable salinity. It is probably highly intolerant of decreases in salinity at the benchmark level. | High | High | Moderate | High |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceIt was assumed for some time that limpets experience anoxia with emergence during every tide, due to the limpets clamping down their shells to avoid desiccation. However, it has been found that limpets will raise their shells to allow transfer of air but at the cost of water loss. Their gills are inefficient at absorbing oxygen from air and limpets are expected to be subject to moderate anaerobic conditions during low tide. In water, limpets can survive at least 18 hours in completely anoxic water by respiring anaerobically (the species studied was Patella caerula) (Santini et al., 2001). Given that limpets do well in areas of organic enrichment (Rogers, 2003; Tablado et al. 1994) which are often hypoxic due to bacterial respiration. Overall, an intolerance of low has been recorded. | Low | High | Low | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidencePatella ulyssiponensis is not known to compete with any non-native species. | No information | Not relevant | No information | Moderate |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThe flesh of Patella ulyssiponensis is highly prized in the Azores and Azorean communities in the USA and, in 1985, was the sixth most important fishery in the Azores (Martins et al., 1987; Corte-Real et al., 2000). Azorean stocks of Patella ulyssiponensis began to decline in the 1970s when snorkel diving became the main means of collection. The fishery was stable as long as recruitment remained high and only individuals 40 mm long were taken so that the limpets matured and bred before extraction. On some of the central islands of the Azores, individuals as small as 20 mm were being taken and the population was in decline because recruitment from outlying populations was low (Martins et al., 1987). A ban on limpet extraction in these areas has probably saved these populations of Patella ulyssiponensis. Therefore Patella ulyssiponensis is probably sensitive to uncontrolled extraction and an intolerance of high has been recorded. | Intermediate | High | Low | High |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidencePatella ulyssiponensis has no known relationships with species subject to extraction fisheries. | No information | Not relevant | No information | Not relevant |
Additional information
Patella ulyssiponensis generally has a high recoverability. After the Torrey Canyon oil spill, the entire populations of certain beaches were wiped out by the uncontrolled use of dispersants. Even so, it took only 3-5 years for Patella ulyssiponensis to return to population sizes and distributions found before the oil spill (Southward & Southward, 1978).Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Patella ulyssiponensis is harvested in the Azores for human consumption of the flesh. Regulation of extraction has prevented local extinction of this species in the Azores but the population has declined (Corte-Real et al., 2001; Martins et al., 1984). Limpets have been suggested as good bioindicators of heavy metal pollution (Bebiano et al., 2003; Catsiki et al., 1991).Bibliography
Bebianno, M.J., Cravo, A., Miguel, C. & Morias, S., 2003. Metallothionein concentrations in a population of Patella aspera: variation with size. Science of the Total Environment, 301, 151-161.
Bonner, T. M., Pyatt, F. B. & Storey, D. M., 1993. Studies on the motility of the limpet Patella vulgata in acidified sea-water. International Journal of Environmental Studies, 43, 313-320.
Catsiki, V.A., Papathanassiou, E. & Bei, F., 1991. Heavy metal levels in characteristic benthic flora and fauna in the central Aegean Sea. Marine Pollution Bulletin, 22, 566-569.
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Corte-Real, H.S.M., Hawkins, S.J. & Thorpe, J.P., 1992. Genetic confirmation that intertidal and subtidal morphs of Patella ulyssiponensis aspera Roding (Mollusca: Gastropoda: Patellidae) are conspecific. Arquipelago, 10, 55-66.
Davies, M.S., 1992. Heavy metals in seawater: effects on limpet pedal mucus production. Water Research, 26, 1691-1693.
Davies, P.S., 1969. Physiological ecology of Patella III. Desiccation effects. Journal of the Marine Biological Association of the United Kingdom, 49, 291-304.
De Pirro, M., Chelazzi, G., Borghini, F. & Focardi, S., 2001. Variations in cardiac activity following acute exposure to copper in three co-occuring but differently zoned Mediterranean limpets. Marine Pollution Bulletin, 42, 1390-1396.
De Pirro, M., Santini, G. & Chelazzi, G., 1999. Cardiac responses to salinity variations in two differently zoned Mediterranean limpets. Journal of Comparative Physiology, B, 169, 501-506.
Delany, J., Myers, A.A. & McGrath, D., 1998. Recruitment, immigration and population structure of two coexisting limpet species in mid-shore tidepools, on the west coast of Ireland. Journal of Experimental Marine Biology and Ecology, 221, 221-230.
Denny, M.A., 1989. Limpet shell shape that reduces drag: laboratory demonstration of a hydrodynamic mechanism and an exploration of its effectiveness in nature. Canadian Journal of Zoology, 67, 2098-2106.
Evans, R.G., 1953. Studies on the biology of British limpets - the genus Patella on the south coast of England. Proceedings of the Zoological Society of London, 123, 357-376.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fretter, V. & Graham, A., 1994. British prosobranch molluscs: their functional anatomy and ecology, revised and updated edition. London: The Ray Society.
Graham, A., 1988. Molluscs: prosobranchs and pyramellid gastropods (2nd ed.). Leiden: E.J. Brill/Dr W. Backhuys. [Synopses of the British Fauna No. 2]
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Jenkins, S.R., Beukers-Stewart, B.D. & Brand, A.R., 2001. Impact of scallop dredging on benthic megafauna: a comparison of damage levels in captured and non-captured organisms. Marine Ecology Progress Series, 215, 297-301. DOI https://doi.org/10.3354/meps215297
Kinne, O. (ed.), 1980. Diseases of marine animals. vol. 1. General aspects. Protozoa to Gastropoda. Chichester: John Wiley & Sons.
Langston, W.J., Chesman, B.S., Burt, G.R., Hawkins, S.J., Readman, J. & Worsfold, P., 2003. Characterisation of European Marine Sites. Poole Harbour Special Protection Area. Occasional Publication. Marine Biological Association of the United Kingdom, 12, 111.
Lincoln, R., Boxshall, G., & Clark, P., 1998. A Dictionary of Ecology, Evolution and Systematics (2nd ed.). Cambridge: Cambridge University Press
Little, C. & Kitching, J.A., 1996. The Biology of Rocky Shores. Oxford: Oxford University Press.
Martins, H.R., Santos, R.S. & Hawkins, S.J., 1987. Exploitation of limpets (Patella spp.) in the Azores with a preliminary analysis of the stocks. International Council for the Exploration of the Sea, Copenhagen ( Denmark). ICES Council Meeting 1987 (collected papers).
Petpiroon, S. & Dicks, B., 1982. Environmental effects (1969 to 1981) of a refinery effluent discharged into Littlewick Bay, Milford Haven. Field Studies, 5, 623-641.
Rogers, K.M., 2003. Stable carbon and nitrogen isotope signatures indicate recovery of marine biota from sewage pollution at Moa Point, New Zealand. Marine Pollution Bulletin, 46, 821-827.
Santini, G., Bruschini, C., Pazzagli, L., Pieraccini, G., Moneti, G. & Chelazzi, G., 2001. Metabolic responses of the limpet Patella caerula (L.) to anoxia and dehydration. Comparative Biochemistry and Physiology, 130A, 1-8.
Shin, P.K.S., 1988. Effects of a spill of bunker oil on the marine biological communities in Hong Kong. Environment International, 14, 545-552.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Tablado, A., Lopez Gappa, J.J. & Magaldi, N.H., 1994. Growth of the pulmonate limpet Siphonaria lessoni (Blainville) in a rocky intertidal area affected by sewage pollution. Journal of Experimental Marine Biology and Ecology, 175, 211-226
Thompson, G.B., 1979. Distribution and population dynamics of the limpet Patella aspera (Lamarck) in Bantry Bay. Journal of Experimental Marine Biology and Ecology, 40, 115-135.
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Citation
This review can be cited as:
Last Updated: 10/08/2004