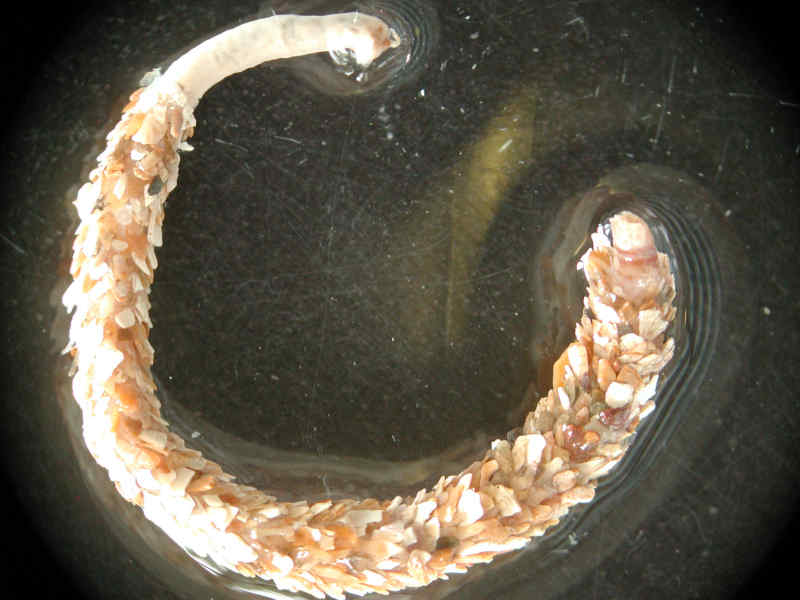
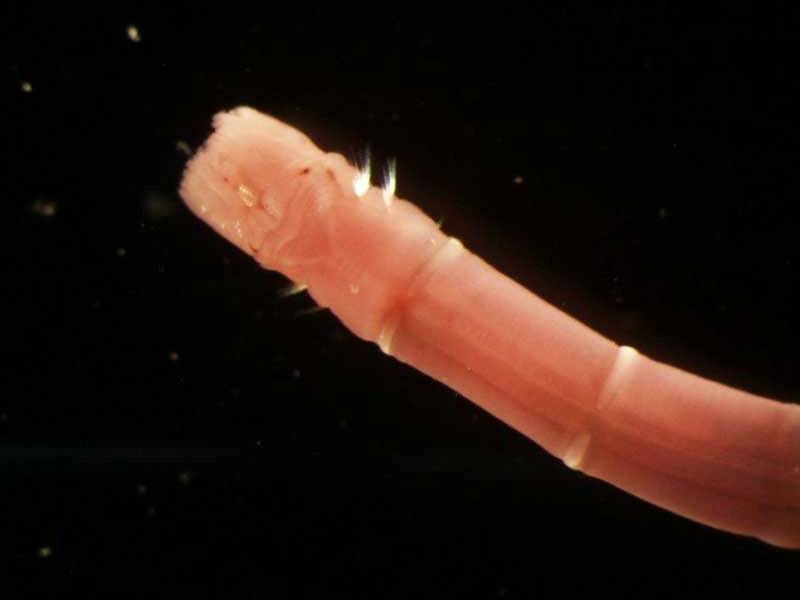
A tubeworm (Owenia fusiformis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Ken Neal & Penny Avant | Refereed by | This information is not refereed |
| Authority | Delle Chiaje, 1844 | ||
| Other common names | - | Synonyms | - |
Summary
Description
Recorded distribution in Britain and Ireland
Widespread around British and Irish coasts.Global distribution
Widely distributed in coastal regions throughout northwest Europe, the Mediterranean, the Indian Ocean and the Pacific.Habitat
Found buried in sand or muddy sand, at or below low water, on fairly sheltered beaches.Depth range
found from the intertidal down to 4,500 mIdentifying features
- A slender tube-dwelling worm up to 10 cm in length.
- Head region with tentacular crown.
- Tube typically with shell fragments arranged like roof tiles.
- Tubes may be so numerous that they bind the sand together, although many may be empty.
- The worm is thin and yellowish or greenish in colour, with small, reddish, frilly lobes at the head end.
Additional information
No text enteredListed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Annelida | Segmented worms e.g. ragworms, tubeworms, fanworms and spoon worms |
| Class | Polychaeta | Bristleworms, e.g. ragworms, scaleworms, paddleworms, fanworms, tubeworms and spoon worms |
| Family | Oweniidae | |
| Genus | Owenia | |
| Authority | Delle Chiaje, 1844 | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 30 - 100mm | ||
| Male size at maturity | 24 - 60mm | ||
| Female size range | 24 - 60mm | ||
| Female size at maturity | |||
| Growth form | Vermiform segmented | ||
| Growth rate | See additional information. | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | |||
| Characteristic feeding method | Active suspension feeder, Surface deposit feeder | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Phytoplankton and particulate organic matter. | ||
| Sociability | |||
| Environmental position | Infaunal | ||
| Dependency | No information found. | ||
| Supports | No information | ||
| Is the species harmful? | No | ||
Biology information
Owenia fusiformis can suspension feed by ciliary filter feeding or in low water flow can deposit feed by bending their flexible tube over so that the feeding crown touches the sediment surface (Rouse & Pleijel, 2001). This polychaete has a lifespan of up to four years in British waters (Rouse & Pleijel, 2001) and has a polymodal population structure of three to five year classes (Menard et al., 1990). The mortality rate increases gradually with age but suddenly increases in the fourth year of life (Menard et al., 1990). Growth is rapid in summer, slows in the autumn and is negligible in winter, resuming in April each year. The maximum recorded density was 4660 individuals per m² but this fluctuated over each year with mortality and massive larval settlement (Menard et al., 1990).Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Offshore seabed, Estuary |
| Biological zone preferences | Bathybenthic (Bathyal), Lower eulittoral, Mid eulittoral, Sublittoral fringe |
| Substratum / habitat preferences | Fine clean sand, Muddy sand, Sandy mud |
| Tidal strength preferences | Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | found from the intertidal down to 4,500 m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
The cosmopolitan nature of Owenia fusiformis has been questioned by Koh & Bhaud (2001) who suggest a review of the Oweniidae because its taxonomy is now very old and more than one species may be included in Owenia fusiformis records.Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | 10,000-100,000 |
| Generation time | 1-2 years |
| Age at maturity | 1 year |
| Season | May - June |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 11-30 days |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
Owenia fusiformis is an annual breeder, gonochoric, with external fertilization (Rouse & Pleijel, 2001). Up to 70,000 oocytes mature from September to April (Gentil et al., 1990) and are spawned during May and June (Rouse & Pleijel, 2001). Oocytes are up to 100 µm in diameter (Rouse & Pleijel, 2001). The sex ratio is female biased and is around 0.86:1. Maturity is size-dependent and all worms 60 mm long or more are mature but some individuals reach maturity at 24 mm. Some individuals may breed in their first year if they can grow fast enough (Gentil et al., 1990). In late May, larval densities can be up to 100,000 per m3 (Thiebaut et al., 1992) and settled densities vary from 4,000 to 15,000 juveniles per m² (Menard et al., 1990).Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceOwenia fusiformis is an infaunal organism and removal of the substratum is likely to also remove adults. Therefore an intolerance of high has been recorded. Due to high fecundity and the prevalence of allochthonous larval supply (Barnay et al., 2003), recovery of a population is likely to occur in less than a year. | High | High | Moderate | Very low |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceOwenia fusiformis in the intertidal and shallow subtidal are likely to be buried as a result of wave action disturbing sediments but can work their way back up to the surface in the flexible tube (Wells et al., 1981). Owenia fusiformis also occurs in areas where dredging spoil is deposited (Dauvin & Gillet, 1991). However, juveniles cannot construct tubes in sediments with a grain size <63 µm. Therefore, if a lot of clay and silt was deposited around a population of Owenia fusiformis recruits will not be able to construct tubes, juvenile mortality will be high, and an intolerance of intermediate has been recorded. | Intermediate | High | Low | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceOwenia fusiformis occurs in front of river outlets (Somaschini, 1993) and in areas where dredging spoil is dumped (Dauvin & Gillet, 1991), and therefore is probably tolerant of an increase in suspended sediment. Owenia fusiformis feeds on suspended organic matter. Therefore an increase in the concentration of phytoplankton and particulate organic matter is likely to be of benefit to Owenia fusiformis, and tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceOwenia fusiformis is a suspension feeder and deposit feeder (Rouse & Pleijel, 2001) but is not reliant on suspended sediment as such and is probably tolerant of a decrease in suspended sediment. | Tolerant | Not relevant | Not sensitive | |
Desiccation [Show more]Desiccation
EvidenceOwenia fusiformis occurs in the intertidal, however, it is infaunal and probably escapes the effects of desiccation due to interstitial water in the fine sediments it inhabits. If desiccation were to occur, intolerance would most likely be intermediate. | Not relevant | Not relevant | Not relevant | Very low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceOwenia fusiformis is mostly found subtidally to abyssal depths but some are found intertidally. An increase in emergence would probably cause those towards the upper limit of distribution to succumb to starvation and/or desiccation. An intolerance of high has been recorded for individuals where emergence is relevant. | High | High | Moderate | Very low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAs Owenia fusiformis is a mainly subtidal species, a decrease in emergence is unlikely to affect it and previously intertidal populations may actually increase in density and tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceIncrease in water flow rate will most likely cause winnowing of the sediment, exposing tubes of Owenia fusiformis. However, Owenia fusiformis is found in front of river outlets in the Mediterranean and can be subject to a wide range of water velocities. The tubes of Owenia fusiformis can stabilize the sediment and reduce water movement related stresses on the benthos (Somaschini, 1993). Owenia fusiformis is probably tolerant to changes in water flow rate. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceA decrease in water flow rate is likely to cause an increase in siltation, however, Owenia fusiformis can migrate up through the sediment in their flexible tube (Wells et al., 1981). However, deposition of sediment with grain sizes <63 µm is likely to cause high mortality amongst recruits which cannot construct tubes in this sort of sediment. An intolerance of intermediate has been recorded to account for recruitment failure in silts and clays. | Intermediate | High | Low | Moderate |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceOwenia fusiformis is a cosmopolitan species and is found in waters from -1 to 30 °C (Dauvin & Thiebaut, 1994) globally. In the Bay of Seine, where there is a large population of Owenia fusiformis, the temperature varies between 5 and 20 °C (Gentil et al., 1990). Some stress may be caused to Owenia fusiformis by temperature changes and an intolerance of low has been recorded. | Low | High | Low | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceOwenia fusiformis is a cosmopolitan species and is found in waters from -1 to 30 °C (Dauvin & Thiebaut, 1994) globally. In the Bay of Seine, where there is a large population of Owenia fusiformis, the temperature varies between 5 and 20 °C (Gentil et al., 1990). Some stress may be caused to Owenia fusiformis due to temperature changes and an intolerance of low has been recorded. | Low | High | Low | Low |
Increase in turbidity [Show more]Increase in turbidity
EvidenceOwenia fusiformis feeds on suspended organic matter and phytoplankton. While an increased turbidity is likely to decrease phytoplankton productivity, it can also feed on organic particulates and is unlikely to be adversely affected. Therefore, tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceA decrease in turbidity is likely to increase phytoplankton productivity and hence potentially augment its food supply. Therefore, tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceWells et al., (1981) reported that Owenia fusiformis in the intertidal and shallow subtidal are likely to be buried as a result of wave action but can survive this by working its way up through the sediment in its flexible tube. However, the effect of being washed out of the sediment by wave action was not commented on. In this situation, Owenia fusiformis would probably have to rebury in the in the sediment and construct a new tube. This is unlikely to occur quickly enough to avoid predation by flatfish and opportunistic predators and intermediate has been recorded to account for the high mortality caused. | Intermediate | High | Low | Very low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceA decrease in wave exposure is likely to cause increased siltation which adult Owenia fusiformis can probably survive (Dauvin & Gillet, 1991; Wells et al., 1981). However, juveniles cannot construct tubes in sediments with a grain size <63 µm. Therefore if there is a lot clay and silt deposited around a population of Owenia fusiformis recruits will not be able to construct tubes, juvenile mortality will be high, and an intolerance of intermediate has been recorded. | Intermediate | Not relevant | NR | Low |
Noise [Show more]Noise
EvidenceOwenia fusiformis can probably detect vibrations in the water and sediment, which may reduce feeding activity but is likely to be tolerant of noise at the benchmark level. | Tolerant | Not relevant | Not sensitive | Very low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceOwenia fusiformis has very simple eyes for light perception and therefore will not be affected by visual disturbance | Tolerant | Immediate | Not sensitive | Very low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceOwenia fusiformis can be up to 10 cm in length (Hayward & Ryland, 1990) and its tubes up to 30 cm in length (Rouse & Pleijel, 2001). Therefore, a passing scallop dredge is likely to remove the anterior end, which can be regenerated (Gibbs et al., 2000), but not the whole worm. An intolerance of low has been recorded to account for this perturbation. | Low | High | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceAdult Owenia fusiformis probably cannot construct new tubes once removed and therefore are probably highly intolerant to displacement. | High | High | Moderate | Very low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceNo information was found on the effect of synthetic compounds on Owenia fusiformis. | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceOwenia fusiformis from the south coast of England were found to have loadings of 1335 µg copper per gram bodyweight and 784 µg zinc per gram bodyweight. The metals were bound in spherules within the cells of the gut (Gibbs et al., 2000). No mention was made of any ill effects of these concentrations of metal within the body and it is presumed that Owenia fusiformis is tolerant of heavy metal contamination. | Tolerant | Very high | Not sensitive | Very low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceA few Owenia fusiformis were recorded in the subtidal sediments of the Pembrokeshire coast after the Sea Empress oil spill but whether densities had increased, decreased or remained the same was not recorded (Rutt et al., 1998). An intolerance to oil cannot be assessed for Owenia fusiformis on the basis of other polychaetes as some are tolerant to oil and others highly intolerant (Kingston et al., 1997). | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information was found on the effect of radionuclides on Owenia fusiformis. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceIncreases in nutrient levels are likely to increase phytoplankton productivity, which would benefit Owenia fusiformis populations. Therefore tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceNo information was found on the effects of hypersalinity on Owenia fusiformis. | No information | Not relevant | No information | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidenceOwenia fusiformis is found in front of river outlets in the Mediterranean (Somaschini, 1993) and English Channel (Gentil et al., 1990) and probably has a low intolerance to decreases in salinity. | Low | High | Low | Low |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceOwenia fusiformis is very tolerant of anoxia and can tolerate anaerobic conditions for up to 21 days by becoming quiescent (Dales, 1958) and therefore is tolerant to changes in oxygenation. | Tolerant | Immediate | Not sensitive | High |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information was found on the effects of microbial pathogens on Owenia fusiformis. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo information was found on the effects of alien species on Owenia fusiformis. | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceOwenia fusiformis is not known to be targeted for extraction. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceTrawls and dredges may remove the anterior end of Owenia fusiformis but the worm regenerates lost ends (Gibbs et al., 2000) and an intolerance of low has been recorded. | Low | Very high | Very Low | Low |
Additional information
Owenia fusiformis has high individual fecundity and high population density. Larval life is long and there is often free exchange of larvae between populations. Spatfall is usually very dense, growth rapid and in optimal conditions, and Owenia fusiformis can reproduce in its first year. Recoverability of this species is likely to be high but variable in rate because wind driven currents and adult fecundity will determine larval supply to defaunated areas.Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
-none-Bibliography
Barnay, A.S., Ellien, C., Gentil, F. & Thiebaut, E., 2003. A model study on variations in larval supply: are populations of the polychaete Owenia fusiformis in the English Channel open or closed? Helgoland Marine Research, 56, 229-237.
Bruce, J.R., Colman, J.S. & Jones, N.S., 1963. Marine fauna of the Isle of Man. Liverpool: Liverpool University Press.
Dales, R.P., 1957. The feeding mechanism and structure of the gut of Owenia fusiformis Delle Chiaje. Journal of the Marine Biological Association of the United Kingdom, 36, 81-89.
Dales, R.P., 1958. Survival of anaerobic periods by two intertidal polychaetes, Arenicola marina (L.) and Owenia fusiformis Delle Chiaje. Journal of the Marine Biological Association of the United Kingdom, 37, 521-529.
Dauvin, J.C. & Gillet, P., 1991. Spatio-temporal variability in population structure of Owenia fusiformis Delle Chiaje (Annelida: Polychaeta) from the Bay of Seine (eastern English Channel). Journal of Experimental Marine Biology and Ecology, 152, 105-122.
Dauvin, J.C. & Thiebaut, E., 1994. Is Owenia fusiformis Delle Chiaje a cosmopolitan species? Memoires du Museum National d'Histoire Naturelle, 162, 383-404.
Fauchald, K., 1977. The polychaete worms. Definitions and keys to the orders, families and genera. USA: Natural History Museum of Los Angeles County.
Gentil, F., Dauvin, J.C. & Menard, F., 1990. Reproductive biology of the polychaete Owenia fusiformis Delle Chiaje in the Bay of Seine (eastern English Channel). Journal of Experimental Marine Biology and Ecology, 142, 13-23.
Gibbs, P.E., Burt, G.R., Pascoe, P.L., Llewellyn, C.A. & Ryan K.P., 2000. Zinc, copper and chlorophyll-derivates in the polychaete Owenia fusiformis. Journal of the Marine Biological Association of the United Kingdom, 80, 235-248.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hayward, P.J. & Ryland, J.S. 1990. The marine fauna of the British Isles and north-west Europe. Oxford: Oxford University Press.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Kingston, P.F., Dixon, I.M.Y., Hamilton, S., Moore, C.G. & Moore, D.C., 1997. Studies on the response of intertidal and subtidal marine benthic communities to the Braer oil spill. In J.M. Davies & G. Topping, (Ed.) The impact of an oil spill in turbulent waters: The Braer. p. 209-253. Edinburgh: Stationary Office.
Koh, B.S. & Bhaud, M., 2001. Description of Owenia gomsoni n. sp. (Oweniidae, Annelida, Polychaeta) from the Yellow Sea and evidence that Owenia fusiformis is not a cosmopolitan species. Vie et Millieu, 51, 77-86.
Menard, F., Gentil, F. & Dauvin, J.C., 1990. Population dynamics and secondary production of Owenia fusiformis Delle Chiaje (Polychaeta) from the Bay of Seine (eastern English Channel). Journal of Experimental Marine Biology and Ecology, 133, 151-167.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Pinedo, S., Sarda, R., Rey, C. & Bhaud, M., 2000. Effect of sediment particle size on recruitment of Owenia fusiformis in the Bay of Blanes (NW Mediterranean Sea): an experimental approach to explain field distribution. Marine Ecology Progress Series, 203, 205-213.
Rouse, G.W. & Pleijel, F., 2001. Polychaetes. New York: Oxford University Press.
Rutt, G.P., Levell, D., Hobbs, G., Rostron, D.M., Bullimore, B., Law, R.J. & Robinson, A.W., 1998. The effects on the marine benthos. In R. Edwards & H. Sime, (Ed.) The Sea Empress oil spill. p.189-206. Chartered Institution of Water and Environmental Management.
Somaschini, A., 1993. A Mediterranean fine-sand polychaete community and the effect of the tube-dwelling Owenia fusiformis Delle Chiaje on community structure. Internationale Revue de Gesamten Hydrobiologie, 78, 219-233.
Thiebaut, E., Dauvin, J.C. & Lagadeuc, Y., 1992. Transport of Owenia fusiformis larvae (Annelida: Polychaeta) in the Bay of Seine. I. Vertical distribution in relation to water column stratification and ontogenic vertical migration. Marine Ecology Progress Series, 80, 29-39.
Wells, R.M.G., Dales, R.P. & Warren, L.M., 1981. Oxygen equilibrium characteristics of the erythrocruorin (extracellular haemoglobin) from Owenia fusiformis Delle Chiaje (Polychaeta: Oweniidae). Comparative Biochemistry and Physiology, A, 70A, 111-114.
Wilson, D.P., 1932. On mitraria larva of Owenia fusiformis Delle Chiaje. Philosophical Transactions of the Royal Society of London, Series B, 221, 231-334.
Yonow, N., 1989. Feeding observations on Acteon tornatilis (Linnaeus) (Opisthobranchia: Acteonidae). Journal of Molluscan Studies, 55, 97-102.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
South East Wales Biodiversity Records Centre, 2018. SEWBReC Worms (South East Wales). Occurrence dataset: https://doi.org/10.15468/5vh0w8 accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 17/04/2008