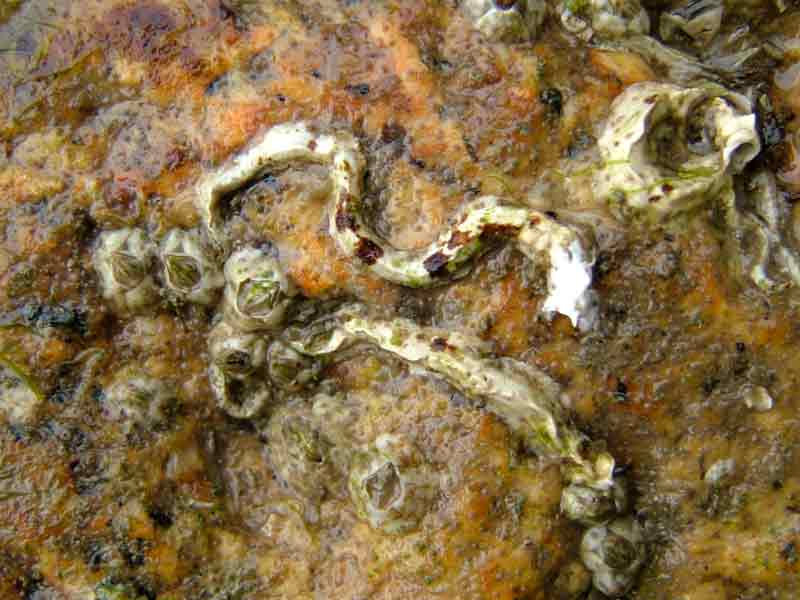
A tubeworm (Spirobranchus triqueter)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Karen Riley & Susie Ballerstedt | Refereed by | This information is not refereed |
| Authority | (Linnaeus, 1758) | ||
| Other common names | - | Synonyms | Pomatoceros triqueter |
Summary
Description
The calcareous tube of Spirobranchus triqueter is 3.5 mm wide and up to 25 mm long. It is white, smooth and irregularly curved with a single, median ridge that ends in a projection over the anterior opening. The operculum bears a shallow, dish-shaped plug (ampulla) which is often conical distally, and may have projections on the crown. The colouration of the body is bright but variable, and the crown of tentacles (radioles) are banded with various colours.
Recorded distribution in Britain and Ireland
Common and widespread on all coasts.Global distribution
Occurs from the coasts of north west Europe to the Mediterranean.Habitat
Spirobranchus triqueter encrusts stones, rocks and shells, and the carapace of some species of decapods. They are predominantly sublittoral to depths of 70 m.Depth range
Up to 70mIdentifying features
- The operculum bears a shallow, dish-shaped plug (ampulla), the distal part is often conical and may have projections on the crown.
- The tube is up to 25 mm long.
- A single ridge runs along the top of the tube, ending in a projection over the anterior opening.
- Colouration of the worm is varied.
Additional information
- May be confused with Spirobranchus lamarcki, the tube of which differs from Spirobranchus triqueter as it has two vestigial ridges, one on each side, in addition to the median keel. Further differences can only be seen when the worm is removed from its tube (Hayward & Ryland, 1995). Further distinction between the two species can be obtained by using biochemical genetics, as described by Ekaratne et al. (1982).
- Males are cream in colour whilst females are bright pink/orange in colour (Thomas, 1940).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Annelida | Segmented worms e.g. ragworms, tubeworms, fanworms and spoon worms |
| Class | Polychaeta | Bristleworms, e.g. ragworms, scaleworms, paddleworms, fanworms, tubeworms and spoon worms |
| Order | Sabellida | |
| Family | Serpulidae | |
| Genus | Spirobranchus | |
| Authority | (Linnaeus, 1758) | |
| Recent Synonyms | Pomatoceros triqueter | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | No information found | ||
| Male size range | up to 25mm | ||
| Male size at maturity | |||
| Female size range | Small(1-2cm) | ||
| Female size at maturity | |||
| Growth form | Vermiform segmented | ||
| Growth rate | 1.5mm/month | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Active suspension feeder | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Plankton and detritus | ||
| Sociability | |||
| Environmental position | Epibenthic | ||
| Dependency | Independent. | ||
| Supports | No information | ||
| Is the species harmful? | No No text entered | ||
Biology information
- Once settled onto the substratum the worm forms a temporary delicate semi-transparent tube, which, when calcareous material is later added at the anterior end (Hayward & Ryland, 1995) dissolves over time (Dons, 1927). The tube is formed by a secretion of calcium carbonate (obtained from seawater) from the collar (Thomas, 1940).
- The growth rate is usually measured by the increase in length of the tube over a period of time. Dons (1927) found that the youngest sessile stages of the animals in Trondheim occurred when the tubes were 800-1200 µm long and the animal was approximately 500 µm in length.
- Hayward & Ryland (1995) and Dons (1927) stated that growth is rapid and sexual maturity is reached in approximately four months. The growth rate has been observed by Dons (1927) to be 1.5 mm per month, although this varied with external conditions. Males and females exhibit the same growth rate (Castric-Fey, 1983). Animals settling during spring show the best growth rate and the rate is greatest during the first year (Castric-Fey, 1983).
- Castric-Fey (1983) reported that the number of segments of the worm increases with age, with a linear relationship being present within the first six months.
- Thomas (1940) reviewed feeding and respiration in the polychaete. Spirobranchus (as Pomatoceros) triqueter never leaves its tube. Occasionally the posterior end of the tube becomes blocked by a calcareous plate with holes in it. Respiration and excretion take place using cilia action to set up currents, bringing water in and down the length of the tube and flushing it back out the same way. Respiration occurs through the surface of the body and the branchial crown.
- Feeding takes place by spreading apart its branchial filaments to expose a central groove. Using cilia action, it induces a current and transports food particles towards its mouth. If particles are too large or too numerous, the tip of a filament bends over and removes it. No sorting of food particles takes place.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Open coast |
| Biological zone preferences | Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Cobbles, Crevices / fissures, Gravel / shingle, Large to very large boulders, Pebbles, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Extremely sheltered, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | Up to 70m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
- Segrove (1941) studied Spirobranchus triqueter in south England and found that there are usually ten times as many males as females present.
- The species has been noted to occur in very exposed to extremely sheltered wave action, very sheltered to exposed water flow rate, and in areas where there is little or no silt present (Price et al., 1980).
- Spirobranchus triqueter is considered to be a primary fouling organism (Crisp, 1965), colonizing artificial commercially important structures such as buoys, ships hulls, docks and offshore oil rigs (OECD, 1967).
- Spirobranchus triqueter is an opportunistic species, making use of available space quickly. In Bantry Bay, south-west Ireland, fouling by the tube worm caused a 65% mortality of scallops and prevented scallops from recolonizing the area after spat collection (Burnell et al., 1991). They also reported that mussel farmers considered that most inner areas of the bay would be subject to this type of fouling.
- Rubin (1985) reported that Spirobranchus (syn. Pomatoceros) triqueter overgrew colonies of encrusting Bryozoa to become the dominant species on experimental panels. However, Bryozoa then grew on the tubes of the species, thereby avoiding exclusion.
- Dominance of Spirobranchus lamarckii over Spirobranchus triqueter is dependent on climatic conditions (Castric-Fey, 1983).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Protandrous hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | No information |
| Generation time | Insufficient information |
| Age at maturity | Approximately 4 months |
| Season | See additional information |
| Life span | See additional information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 11-30 days |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | See additional information |
Life history information
- Male Spirobranchus triqueter release spermatogonia or primary spermatocytes and females release primary oocytes through a pair of gonoducts, consisting of a ciliated funnel and tube (Thomas, 1940).
- Hayward & Ryland (1995) and Segrove (1941) suggested that breeding of Spirobranchus triqueter probably takes place throughout the year. However, Hayward & Ryland (1995) noted a breeding peak in spring and summer and records from Port Erin by Moore (1937) indicated that breeding only took place in April in this location.
- Castric-Fey (1983) studied variations in settlement rate and concluded that, although the species settled all year round, very rare settlement was observed during winter and maximum settlement occurred in April, June, August and Sept-Oct. Studies in Bantry Bay (Cotter et al., 2003) revealed a single peak in recruitment during summer (especially July and August) with very little recruitment at other times of the year. More individuals settled on panels at 7 m than at 4 m.
- Larvae are pelagic for about 2 to 3 weeks in the summer. However, in the winter this amount of time increases to about two months (Hayward & Ryland, 1995).
- Longevity has been recorded to be between 1.5 to 4 years. Hayward & Ryland (1995) noted that individuals lived approximately 1.5 years, with most individuals dying after breeding (Hayward & Ryland, 1995). Castric-Fey (1983) found that under laboratory conditions, individuals were still alive after 2.5 years. However, Castric-Fey (1983) also stated that under natural conditions it is probable that they do not live any longer than this. Whilst Dons (1927) found that, according to measured growth rate, some of the individuals he studied would have been at least four years old.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidencePomatoceros triqueter is attached permanently to rocks, boulders or shingle. Removal of substratum will remove calcareous tubes and animals contained in them. Intolerance is assessed as high. Recoverability is likely to be high (see additional information below). | High | High | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering with a 5 cm layer of sediment would completely cover the tubes of Pomatoceros triqueter that usually lie flat against the surface of the rock. It is also likely that too much sediment on the surface of rocks or shells would prevent settlement of larvae and impair the long term survival of populations. Intolerance has been assessed to be high. Recoverability is likely to be high (see additional information below). | High | High | Moderate | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceRecords show confusion as to whether Pomatoceros triqueter is tolerant of high suspended sediment levels. According to Bacescu (1972), sabellids are accustomed to turbidity and silt. Stubbings & Houghton (1964) found Pomatoceros triqueter in Chichester harbour, a muddy harbour, therefore agreeing with the previous statement. However, Pomatoceros triqueter has been noted to occur in areas where there is little or no silt present (Price et al., 1980) and according to Lewis (1957), Pomatoceros triqueter is highly susceptible to unfavourable conditions, always requiring stability and clean water. Moore (1937) and Nair (1962) agreed with this. However, Pomatoceros triqueter has been recorded in areas where suspended sediment levels can be high; demonstrating that it can tolerate high suspended sediment concentrations. A supply of suspended sediment will probably also be important to Pomatoceros triqueter because the species requires a supply of particulate matter for suspension feeding. At the benchmark level of an increase of 100mg/l for one month, the likely impact would be an increase in cleaning costs. Intolerance has been assessed as low. Recoverability is likely to be high. | Low | High | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidencePomatoceros triqueter has been noted to occur in areas where there is little or no silt present (Price et al., 1980). The species is an active suspension feeder and will probably not be highly intolerant of suspended sediment concentrations. As an energetic cost would probably be entailed to create currents to transport food particles, intolerance has been assessed to be low. On return to normal conditions, recoverability is likely to be high. | Low | High | Low | Moderate |
Desiccation [Show more]Desiccation
EvidenceAs Pomatoceros triqueter occurs in the subtidal region it will be tolerant to a certain amount of desiccation. The species probably survives by closing the operculum of the tube, however, the amount of time available for feeding and respiration will be reduced, and therefore the population's viability may be reduced. Some individuals are also likely to die. Intolerance has been assessed to be intermediate. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAn increase in the emergence regime will increase the amount of time some individuals are exposed to air. At the benchmark level of an increase of one hour over the period of a year, those higher on shore are likely to die. Intolerance has been assessed to be intermediate. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA decrease in the emergence regime may mean that more time can be spent feeding, but is unlikely to have any adverse effects. Therefore Pomatoceros triqueter is likely to tolerate a decrease in emergence, and may actually benefit. | Tolerant* | Not relevant | Not sensitive* | Not relevant |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidencePomatoceros triqueter has been noted to occur in areas with very sheltered to exposed water flow rates (Price et al., 1980). Wood (1988) observed Pomatoceros sp. in strong tidal streams and Hiscock (1983) found that in strong tidal streams or strong wave action where abrasion occurs, fast growing species such as Pomatoceros triqueter occur. Therefore, the species is probably tolerant of an increase in water flow rate, and the species may actually increase in abundance. | Tolerant* | Not relevant | Not sensitive* | Not relevant |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidencePomatoceros triqueter has been noted to occur in areas with very sheltered to exposed water flow rates (Price et al., 1980). The species has been assessed to be tolerant. | Tolerant | Not relevant | Not sensitive | Not relevant |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceMaximum sea surface temperatures around the British Isles rarely exceed 20 °C (Hiscock, 1998) and, as Pomatoceros triqueter occurs as far south as the Mediterranean, it will therefore be subject to a wider range of temperatures than experienced in the British Isles. Further information also backs this up. Castric-Fey (1983) found that animals settling during spring showed the best growth rate and the best larval settlement occurred in the summer months. Pomatoceros triqueter has been assessed as tolerant* to an increase in temperature. | Tolerant* | Not relevant | Not sensitive* | Not relevant |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceMinimum surface sea water temperatures rarely fall below 5 °C around the British Isles (Hiscock, 1998). Below a temperature of 7°C Pomatoceros triqueter is unable to build calcareous tubes (Thomas, 1940). This means that, although adults may be able to survive a decrease in temperature, larvae would not be able to attach to the substratum. Intolerance has been assessed to be intermediate. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Moderate |
Increase in turbidity [Show more]Increase in turbidity
EvidenceAccording to Bacescu (1972), sabellids are accustomed to turbidity and silt. Pomatoceros triqueter has also recently been recorded by De Kluijver (1993) from Scotland in the aphotic zone, indicating that the species would not be sensitive to an increase in turbidity. | Tolerant | Not relevant | Not sensitive | Not relevant |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceAccording to Bacescu (1972), sabellids are accustomed to turbidity and silt. According to Lewis (1957), Pomatoceros triqueter is highly susceptible to unfavourable conditions, always requiring stability and clean water. Moore (1937) and Nair (1962) agreed with this. Therefore, Pomatoceros triqueter is unlikely to be sensitive to a decrease in turbidity. | Tolerant | Not relevant | Not sensitive | Not relevant |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidencePomatoceros triqueter has been noted to occur in areas with variable wave action; extremely sheltered to very exposed (Price et al., 1980). The hard calcareous tube is resistant to abrasion from sand, gravel and boulders (Wood, 1988; Hiscock, 1983) that are mobilised by wave action. With an increase in wave exposure over a period of a year the viability of the population may be reduced due to a reduction in feeding and larval settlement. Therefore intolerance of Pomatoceros triqueter to an increase in wave exposure is likely to be low. On return to normal conditions, recoverability is likely to be high. | Low | High | Low | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidencePomatoceros triqueter has been noted to occur in areas with variable wave action; extremely sheltered to very exposed (Price et al., 1980). As the species can tolerate very low wave exposure, it is therefore probably tolerant of a decrease in wave exposure. | Tolerant | Not relevant | Not sensitive | Not relevant |
Noise [Show more]Noise
EvidencePolychaetes may be able to detect vibration, and withdraw into their tube. However, at the benchmark level the species is unlikely to be sensitive to noise. | Tolerant | Not relevant | Not sensitive | Not relevant |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceShadows detected by the photoreceptive surface of serpulid polychaetes may result in withdrawal of the worm back into its tube (Kinne, 1970). However, at the benchmark level the species is unlikely to be sensitive to visual presence. | Tolerant | Not relevant | Not sensitive | Not relevant |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidencePomatoceros triqueter has a hard calcareous tube that is resistant to sand and gravel abrasion (Wood, 1988). Hiscock (1983) noted that a community, under conditions of scour and abrasion from stones and boulders moved by storms, developed into a community consisting of fast growing species such as Pomatoceros triqueter. Off Chesil Bank, the epifaunal community dominated by Pomatoceros triqueter, Balanus crenatus and Electra pilosa, decreased in cover in October, was scoured away in winter storms, and was recolonized in May to June (Warner, 1985). Warner (1985) reported that the community did not contain any persistent individuals, being dominated by rapidly colonizing organisms. But, while larval recruitment was patchy and varied between the years studied, recruitment was sufficiently predictable to result in a dynamic stability and a similar community was present in 1979, 1980, and 1983. Scour due to winter storms is probably greater than the benchmark level. Scour and abrasion will probably remove a proportion of the population, suggesting an intolerance of intermediate. However, it demonstrates rapid growth and recruitment so that it is not considered to be sensitive. The abundance of Pomatoceros triqueter may increase due to decreased competition from other species. | Intermediate | High | Low | Not relevant |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceIf tubes containing the worm are removed, the tubes will not be able to be reattached to the substratum surface. However, Thomas (1940) found that if Pomatoceros triqueter is removed from its tube, it will start to make a new one in a few hours. Therefore, it is likely that the worm will be able to leave the old tube to start constructing another. This would probably involve an added energetic cost, therefore population viability may be affected. Intolerance has been assessed to be low. Recoverability is likely to be high. | Low | High | Low | High |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceThere is insufficient information to assess the intolerance of Pomatoceros triqueter to synthetic chemicals. | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryan (1984) suggested that, on evidence available for several species, that polychaetes are fairly resistant to heavy metals. However, there is insufficient information available to assess intolerance of Pomatoceros triqueter to heavy metal contamination. | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceLarge numbers of dead polychaetes and other fauna were washed up at Rulosquet marsh near Isle de Grand following the Amoco Cadiz oil spill in 1978 (Cross et al., 1978). However, no information was found relating to Pomatoceros triqueter in particular. Therefore, insufficient information was available to assess the species intolerance. | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceThere is insufficient information to assess the intolerance of Pomatoceros triqueter to radionuclides. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceThere is insufficient information to assess the intolerance of Pomatoceros triqueter to nutrient levels. | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidencePomatoceros triqueter occurs in fully saline waters and is probably relatively tolerant of an increase in salinity. | Tolerant | Not relevant | Not sensitive | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidencePomatoceros triqueter occurs in fully saline coastal waters and has not been recorded from brackish or estuarine waters. Therefore, it is likely that the species will be very intolerant of a decrease in salinity. However, Dixon (1985) views the species as able to withstand significant reductions in salinity. The degree of reduction in salinity and time that the species could tolerate those levels were not recorded. Therefore, there is insufficient information available to assess the intolerance of Pomatoceros triqueter to a reduction in salinity. | High | High | Moderate | Low |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceCole et al. (1999) suggest possible adverse effects on marine species below 4 mg/l and probable adverse effects below 2 mg/l. However, no information was found relating to intolerance of Pomatoceros triqueter to oxygen levels. Insufficientinformation was available to assess intolerance of the species at the benchmark level of 2 mg/l for a week. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceThomas (1940) recorded parasites of Pomatoceros triqueter. Trichodina pediculus (a ciliate) was observed in fair numbers moving over the branchial crown. However, this is a commensal, not a parasite. Parasites found in the worm include gregarines & ciliated protozoa and parasites that had the appearance of sporozoan cysts. However, no information was found about the effects of microbial pathogens on Pomatoceros triqueter. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceAlthough several species of serpulid polychaetes have been introduced into British waters none are reported to compete with Pomatoceros triqueter (Eno et al., 1997). | Tolerant | Not relevant | Not sensitive | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNo extraction of Pomatoceros triqueter is known to occur. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceNo extraction of other species is likely to have any effect on Pomatoceros triqueter. | Not relevant | Not relevant | Not relevant | Not relevant |
Additional information
The species is fairly widespread, reaches sexual maturity within 4 months (Hayward & Ryland, 1995; Dons, 1927) and longevity has been recorded to be between 1.5 and 4 years (Hayward & Ryland, 1995; Castric-Fey, 1983; Dons, 1927). Larvae are pelagic for about 2-3 weeks in the summer and about 2 months in the winter (Hayward & Ryland, 1995), enabling them to disperse widely. Recovery is therefore likely to be high.Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Pomatoceros triqueter is an opportunistic species that can live on a variety of substrates; from rocks, boulders and pebbles to man-made structures. The fouling of the tube worm can compete with and exclude other species.Bibliography
Bacescu, M.C., 1972. Substratum: Animals. In: Marine Ecology: A Comprehensive Treatise on Life in Oceans and Coastal Waters. Volume 1 Environmental Factors Part 3. (ed. O. Kinne ). Chichester: John Wiley & Sons.
Bradshaw, C., Veale, L.O., Hill, A.S. & Brand, A.R., 2002. The role of scallop-dredge disturbance in long-term changes in Irish Sea benthic communities: a re-analysis of an historical dataset. Journal of Sea Research, 47, 161-184. DOI https://doi.org/10.1016/S1385-1101(02)00096-5
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Burnell, G.M., Barnett, M., O'Caroll, T. & Roantree, V., 1991. Scallop spat collection and on-growing trials in south-west Ireland. Actes de Colloques. Institutes Francais de Recherche pour l'Exploitation de la Mer (IFREMER), 17, 139-144.
Castric-Fey, A., 1983. Recruitment, growth and longevity of Pomatoceros triqueter and Pomatoceros lamarckii (Polychaeta, Serpulidae) on experimental panels in the Concarneau area, South Brittany. Annales de l'Institut Oceanographique, Paris, 59, 69-91.
Cole, S., Codling, I.D., Parr, W. & Zabel, T., 1999. Guidelines for managing water quality impacts within UK European Marine sites. Natura 2000 report prepared for the UK Marine SACs Project. 441 pp., Swindon: Water Research Council on behalf of EN, SNH, CCW, JNCC, SAMS and EHS. [UK Marine SACs Project.]. Available from: http://ukmpa.marinebiodiversity.org/uk_sacs/pdfs/water_quality.pdf
Cotter, E., O’Riordan, R.M. & Myers, A.A., 2003. Recruitment patterns of serpulids (Annelida: Polychaeta) in Bantry Bay, Ireland. Journal of the Marine Biological Association of the United Kingdom, 83 (1), 41- 48. DOI https://doi.org/10.1017/S0025315403006787h
Crisp, D.J., 1965. The ecology of marine fouling. In: Ecology and the Industrial Society, 5th Symposium of the British Ecological Society, 99-117 (ed. G.T. Goodman, R.W. Edwards & J.M. Lambert).
Cross, F.A., Davis, W.P., Hoss, D.E. & Wolfe, D.A., 1978. Biological Observations, Part 5. In The Amoco Cadiz Oil Spill - a preliminary scientific report (ed. W.N.Ness). NOAA/EPA Special Report, US Department of Commerce and US Environmental Protection Agency, Washington.
De Kluijver, M.J., 1993. Sublittoral hard-substratum communities off Orkney and St Abbs (Scotland). Journal of the Marine Biological Association of the United Kingdom, 73 (4), 733-754.
Dixon, D.R., 1985. Cytogenetic procedures. Pomatoceros triqueter: A test system for environmental mutagenesis. In The effects of stress and pollution in marine animals.
Dons, C., 1927. Om Vest og voskmåte hos Pomatoceros triqueter. Nyt Magazin for Naturvidenskaberne, LXV, 111-126.
Ekaratne, K., Burfitt, A.H., Flowerdew, M.W. & Crisp, D.J., 1982. Separation of the two Atlantic species of Pomatoceros, P. lamarckii and P. triqueter (Annelida: Serpulidae) by means of biochemical genetics. Marine Biology, 71, 257-264.
Eno, N.C., Clark, R.A. & Sanderson, W.G. (ed.) 1997. Non-native marine species in British waters: a review and directory. Peterborough: Joint Nature Conservation Committee.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Hiscock, K., ed. 1998. Marine Nature Conservation Review. Benthic marine ecosystems of Great Britain and the north-east Atlantic. Peterborough, Joint Nature Conservation Committee.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Kinne, O. (ed.), 1970. Marine Ecology: A Comprehensive Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors Part 1. Chichester: John Wiley & Sons
Lewis, J.R., 1957. Intertidal communities of the northern and western coasts of Scotland. Transactions of the Royal Society of Edinburgh, 63, 185-220.
Moore, H.B., 1937. Marine Fauna of the Isle of Man. Liverpool University Press.
Nair, N.B., 1962. Ecology of marine fouling and wood-boring organisms of western Norway. Sarsia, 8, 1-88.
OECD (ed.), 1967. Catalogue of main marine fouling organisms. Vol. 3: Serpulids. Paris: Organisation for Economic Co-operation and Development.
Price, J.H., Irvine, D.E. & Farnham, W.F., 1980. The shore environment. Volume 2: Ecosystems. London Academic Press.
Rubin, J.A., 1985. Mortality and avoidance of competitive overgrowth in encrusting Bryozoa. Marine Ecology Progress Series, 23, 291-299.
Segrove, F., 1941. The development of the serpulid Pomatoceros triqueta L. Quarterly Journal of Microscopical Science, 82, 467-540.
Stubbings, H.G. & Houghton, D.R., 1964. The ecology of Chichester Harbour, south England, with special reference to some fouling species. Internationale Revue der Gesamten Hydrobiologie, 49, 233-279.
Thomas, J.G., 1940. Pomatoceros, Sabella and Amphitrite. LMBC Memoirs on typical British marine plants and animals no.33. University Press of Liverpool. Liverpool
Wood, E. (ed.), 1988. Sea Life of Britain and Ireland. Marine Conservation Society. IMMEL Publishing, London
Datasets
Bristol Regional Environmental Records Centre, 2017. BRERC species records recorded over 15 years ago. Occurrence dataset: https://doi.org/10.15468/h1ln5p accessed via GBIF.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. IBIS Project Data. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Lancashire Environment Record Network, 2018. LERN Records. Occurrence dataset: https://doi.org/10.15468/esxc9a accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
Merseyside BioBank., 2018. Merseyside BioBank Active Naturalists (unverified). Occurrence dataset: https://doi.org/10.15468/smzyqf accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
North East Scotland Biological Records Centre, 2017. NE Scotland other invertebrate records 1800-2010. Occurrence dataset: https://doi.org/10.15468/ifjfxz accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
South East Wales Biodiversity Records Centre, 2018. SEWBReC Worms (South East Wales). Occurrence dataset: https://doi.org/10.15468/5vh0w8 accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. Dr Mary Gillham Archive Project. Occurance dataset: http://www.sewbrec.org.uk/ accessed via NBNAtlas.org on 2018-10-02
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 26/10/2005