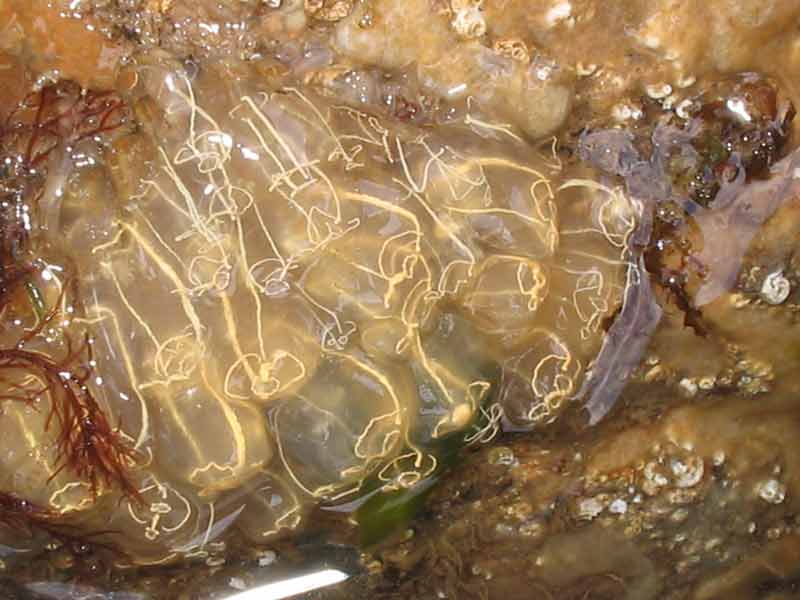
Light bulb sea squirt (Clavelina lepadiformis)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Karen Riley | Refereed by | Dr Xavier Turon |
| Authority | (Müller, 1776) | ||
| Other common names | - | Synonyms | - |
Summary
Description
Clavelina lepadiformis is a colonial sea squirt that grows up to 2 cm high. Groups of transparent zooids are joined at the base by short stolons. Eggs and larvae vary in colour and are visible in the atrial cavity. In the Mediterranean, the eggs and embryos are most often yellowish white and sometimes pink (X. Turon, pers. comm.) although in other areas in NW Europe they can also be red (Fish & Fish, 1996). Zooids possess a white ring around the pharynx and have pale yellow or white longitudinal lines along the endostyle and dorsal lamina, which gives this species its 'light-bulb' appearance. In some areas, colonies regress in winter and re-grow in spring although in the Mediterranean this may not be the case. De Caralt et al. (2002) looked at the differences in Clavelina lepadiformis between populations inside and outside of harbours and found that the population inside the harbour remained all year (albeit often at very low abundances). In contrast, the population in a rocky littoral area outside the harbour was aestivated (regressed) for up to seven months over the summer period (De Caralt et al., 2002).
Recorded distribution in Britain and Ireland
Clavelina lepadiformis occurs around most coasts of Britain and Ireland.
Global distribution
Its distribution extends from southern Norway to the Mediterranean.
Habitat
Clavelina lepadiformis attaches itself to rocks, stones and seaweed in the sublittoral, to a depth of about 50 m.
Depth range
Down to 50 mIdentifying features
- Colonies of individual, transparent zooids are attached to one another at the base by stolons.
- Zooids possess a white ring around the pharynx and have pale yellow or white longitudinal lines along the endostyle and dorsal lamina.
- Siphons are close together and the pharyngeal region is short.
- The gut loop is below the branchial sac, and zooids are taller than wide.
- Reaches a maximum height of 2 cm.
Additional information
The light bulb sea squirt attaches itself to rocks, stones and seaweed in the sublittoral, down to a depth of about 50 m. Individual zooids are small in spring growing to full size by about the end of May in Britain.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Chordata | Sea squirts, fish, reptiles, birds and mammals |
| Class | Ascidiacea | Sea squirts |
| Order | Aplousobranchia | |
| Family | Clavelinidae | |
| Genus | Clavelina | |
| Authority | (Müller, 1776) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | Up to 2 cm | ||
| Male size at maturity | |||
| Female size range | Small (1-2cm) | ||
| Female size at maturity | |||
| Growth form | Cylindrical | ||
| Growth rate | See additional information. | ||
| Body flexibility | Low (10-45 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Active suspension feeder, See additional information | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Suspended detritus and plankton | ||
| Sociability | Colonial | ||
| Environmental position | Epibenthic | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No Clavelina lepadiformis has been noted to be markedly toxic towards invertebrate larvae and bacteria (Teo & Ryland, 1995). Extracts of the species produced high mortality in invertebrates experimentally (Teo & Ryland, 1994). The species is also known to contain the cytotoxic alkaloid, lepadin A (Steffan, 1991). Tarjuelo et al. (2002) studied the defence mechanisms of six species of colonial ascidians and found Clavelina lepadiformis to be the least palatable when pieces of the tunic and zooid were offered to predators, Mature larvae were also reported to be highly unpalatable (Tarjuelo et al., 2002). | ||
Biology information
- The light bulb sea squirt grows to a maximum height of 20 mm (Fish & Fish, 1996; Picton, 1997).
- Colonies grow rapidly in spring and are full size after about two months (K. Hiscock, pers. comm.).
- The growth rate for settled specimens of Clavelina lepadiformis was found to be high (Tursi et al., 1977), although measures of growth rate were not found.
- Clavelina lepadiformis is an active suspension feeder, feeding on suspended detritus and plankton present in water passing through the branchial basket (Fish & Fish, 1996). It actively pumps water and can therefore thrive in very still conditions. The structure of the branchial sac for Clavelina lepadiformis is in its simplest form; the gill sheet is formed by a single screen with slits (Fiala-Medioni, 1978). Fiala-Medioni (1974) showed that filtration efficiency decreased with an increase in simplicity of this structure.
- The zooids of Clavelina lepadiformis are seldom fouled, other than at the base, either because of possible chemical defences or because of the delicate texture of its tunic (Teo & Ryland, 1994).
- Predators include bottom-feeding fish, carnivorous gastropods and starfish (Millar, 1970). Flatworms are also predators, Prostheceraeus moseleyi being a significant predator of Clavelina lepadiformis is the Mediterranean (X. Turon, pers. comm.).
A study by de Caralt et al. (2002) revealed significant differences in certain aspects of the biology of Clavelina lepadiformis between harbour and open rocky littoral populations in the Mediterranean. Although no morphological differences were found, the abundance in the harbour populations were an order of magnitude higher than at the open littoral population. Furthermore, the harbour population did not experience aestivation (a period of inactivity and reduced metabolic activity), unlike the rocky littoral population, and reproduction also varied greatly. The littoral population only produced larvae for 2-3 months over winter and only had one gonadal cycle per year. By contrast, larvae were present in the harbour population from November to June with several gonadal cycles within this time. They concluded that there was marked ecotypic variation between populations of both habitat types and that the harbour population showed more opportunistic traits (Caralt et al., 2002).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Estuary, Offshore seabed, Open coast, Strait or Sound |
| Biological zone preferences | Lower infralittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Large to very large boulders, Overhangs, Pebbles, Small boulders |
| Tidal strength preferences | |
| Wave exposure preferences | Exposed, Extremely sheltered, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | Down to 50 m |
| Other preferences | |
| Migration Pattern | Non-migratory or resident |
Habitat Information
- The species is absent in the Bristol Channel, between Morecambe Bay and Colwyn Bay on the west coast of England, between the Firth of Forth and Newcastle upon Tyne, and the Humber Estuary and Dover on the east coast of Britain. It also has a variable abundance in Ireland.
- Clavelina lepadiformis is a very common shallow water sea squirt that is usually found on vertical rock faces and on the sides of boulders, to about 50 m depth down (Picton & Costello, 1998; Berrill, 1950). It is also found on shells, stones and seaweeds (Picton & Costello, 1998), is a typical species of harbour areas, commonly found growing on artificial surfaces.
- Naranjo et al. (1996) found that the light bulb sea squirt preferred light, shallow environments and was tolerant of salinities as low as 14 psu (Fish & Fish, 1996). It occurs in a wide range of exposure, but is most abundant in moderately exposed sites in the infralittoral zone (Picton, 1997).
- Naranjo et al. (1996) found that the species was dominant in a low rate of water renewal, excess silting and high suspended solid concentrations, although the species also occurred in other more wave exposed sites.
- In a study comparing Mediterranean and Atlantic populations of Clavelina lepadiformis in interior (harbours, marinas and fjords) and exterior (open rocky littoral) areas, Turon et al. (2003) found strong evidence that the interior Mediterranean clade (group of organisms sharing the same common ancestry) originated from the Atlantic clade. The Atlantic forms were not found to be divided between interior and exterior clades (Turon et al., 2003).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Budding |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | 11-100 |
| Generation time | <1 year |
| Age at maturity | 2-3 months |
| Season | June - September |
| Life span | 1-2 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Ovoviviparous |
| Duration of larval stage | 2-10 days |
| Larval dispersal potential | 100 -1000 m |
| Larval settlement period | Late summer |
Life history information
Sea squirts are permanent hermaphrodites that undergo both sexual and asexual reproduction.
Sexual Reproduction. Fish & Fish (1996) state that it is not easy to determine the age of ascidians, particularly that of colonial forms but that the lifetime is probably around one or two years. Each zooid reproduces sexually once, with the production of eggs possibly going on for weeks or months (Berrill, 1975). Breeding tends occur during June to September in temperate and cold seas (Picton, 1997; Millar, 1970), but in tropical waters it may continue throughout the year (Millar, 1970). In the Mediterranean, the breeding season is winter/spring (X. Turon, pers. comm.). Fertilization takes place internally, in the atrium, where development into the tadpole larvae stage also takes place (Fish & Fish, 1996; Berril, 1950). This process is most likely to occur by cross-fertilization. Brunetti (1987) recorded up to about 50 embryos present in the atrium at one time whereas Tarjuelo & Turon (2004) gave an estimate of 66 embryos. Clavelina lepadiformis brood a large number of small undifferentiated larvae (Tarjuelo & Turon, 2004). After release, the larvae are free-swimming for about three hours (Fish & Fish, 1996; Brunetti, 1987). After this time the larvae settle on suitable substratum and metamorphosis into an adult sea squirt takes place. Development of the oozoid takes up to 3 days, and after 2-3 months of post-developmental growth they become sexually mature (Berrill, 1950).
Asexual reproduction. Clavelina lepadiformis undergoes stolonic asexual budding. At the end of the sexual breeding season, towards the end of the summer, zooids disappear or are resorbed. Over the winter period the colony survives as 'winter buds' from which new zooids develop in spring (Berrill, 1950; Fish & Fish, 1996; Picton & Costello, 1998). In the winter months, when the zooids undergo de-differentiation, the resulting cylindrical bodies of many species of Clavelinidae are often found on rocky shores (Millar, 1970). In the Mediterranean the species reproduces in winter/spring and aestivates (aestivation is a period of inactivity or reduced metabolic activity) in summer (X. Turon, pers. comm.).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceClavelina lepadiformis is permanently attached to the substratum. Removal of substratum will result in loss of the population. Adults are unlikely to be able to reattach, therefore recovery depends upon dispersal of larvae during the breeding season. Intolerance to substratum loss is assessed as high. Recoverability is likely to be moderate (see Additional Information below). | High | Moderate | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceClavelina lepadiformis reaches up to 20 mm in height and often colonizes vertical surfaces and overhangs. Smothering by 5 cm depth of sediment will completely cover the majority of the population, with only those colonizing overhangs and vertical surfaces not being affected. Therefore, intolerance is assessed as high. Recoverability is likely to be moderate (see Additional Information below). | High | Moderate | Moderate | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceIncreased siltation can cause clogging of ascidians respiratory organs (Bakus, 1968). Although Clavelina lepadiformis has relatively wide apertures which help prevent clogging from particles (Naranjo et al., 1996), the structure of its branchial sac is in its simplest form; a gill sheet is formed by a single screen with slits (Fiala-Medioni, 1978). This means that they are less efficient in expelling particles, and more likely to suffer from clogging of feeding apparatus than other forms of sea squirts, such as Ciona intestinalis. Nevertheless, Naranjo et al. (1996) found that Clavelina lepadiformis was dominant in a low rate of water renewal, excess silting and high suspended solid concentrations Therefore, intolerance to siltation is assessed as intermediate. Recoverability is likely to be high (see Additional Information below). | Intermediate | High | Low | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details Evidence | No information | |||
Desiccation [Show more]Desiccation
EvidenceThe sea squirt is a delicate animal, so exposure to desiccating influences for one hour will probably result in death of a proportion of the population. Therefore, intolerance is assessed as high. Recoverability is likely to be moderate (see Additional Information below). | High | Moderate | Moderate | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceClavelina lepadiformis is a sublittoral species. Changes in the emergence regime, where the lower shore becomes exposed for longer, are likely to result in a proportion of the population dying. Intolerance to the emergence regime is assessed as high. Recoverability is likely to be moderate (see Additional Information below). | High | Moderate | Moderate | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceClavelina lepadiformis thrives in areas where there is very little, if any, water movement (for instance, Abereiddy Quarry, Pembrokeshire (Hiscock & Hoare, 1975) as it is an active suspension feeder. Naranjo et al. (1996) found that the species was dominant in a low rate of water renewal, excess silting and high suspended solid concentrations. High water flow rates may be detrimental to feeding ability and posture but are unlikely to cause detachment. On resumption of normal energy expenditure, condition should be restored rapidly. Intolerance is assessed as low. Recoverability is likely to be very high (see Additional Information below). | Low | Very high | Very Low | Moderate |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details Evidence | No information | |||
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceAs the breeding season is temperature dependant (Berrill, 1975; Millar, 1970), a change in temperature is likely to result in a change in its time and duration. The distribution of the light bulb sea squirt extends to waters which lie to the north and south of Britain, and therefore to lower and higher temperatures. Long term changes in temperature can probably be accommodated, whereas acute short term changes may cause a proportion of the population to die. During the severe winter of 1962-63, although no significant mortality was noted, Crisp et al. (1964) found that many compound ascidians were retarded in renewal of the colony after 'winter budding', and some individuals may have been killed. Intolerance is assessed as intermediate. Recoverability is likely to be high (see Additional Information below). | Intermediate | High | Low | Moderate |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details Evidence | No information | |||
Increase in turbidity [Show more]Increase in turbidity
EvidenceThe species is frequently dominant in areas such as harbours with high levels of suspended solids and low light penetration. Naranjo et al. (1996) found that the species was dominant in a low rate of water renewal, excess silting and high suspended solid concentrations. However, Moore (1977) found populations of Clavelina lepadiformis replacing Botryllus schlosseri in clear water creeks near West Mersea. It is therefore likely that Clavelina lepadiformis is tolerant to changes in turbidity. A number of ascidians have been shown to spawn in response to a sharp increase in light (Berrill, 1975; Whittingham, 1967). Therefore, it is possible that spawning in Clavelina lepadiformis could be triggered by low turbidity. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
Evidence | No information | |||
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceClavelina lepadiformis is tolerant of a wide range of exposure, but is most abundant in moderately exposed sites (Picton, 1997). Therefore, changes in wave exposure are not likely to have any significant effect. However, increases above moderately exposed are likely to have adverse effects on the population resulting in loss of colonies, therefore, intolerance is assessed as intermediate. Recoverability is likely to be high (see additional Information below). | Intermediate | High | Low | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details Evidence | No information | |||
Noise [Show more]Noise
EvidenceIt is unlikely that sea squirts can detect noise vibrations. | Tolerant | Not relevant | Not sensitive | Low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceIt is unlikely that sea squirts are able to detect visual presence or lower light intensities due to shading. | Tolerant | Not relevant | Not sensitive | Low |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceThe light bulb tunicate is permanently attached to the substratum and is unable to move out of the way from abrasive objects. The body of the species is soft and delicate, so abrasion is likely to cause physical damage and possibly death. Intolerance is assessed as high. Recoverability is likely to be moderate (see additional information below). | High | Moderate | Moderate | High |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceAfter displacement, adults may not be able to reattach to the substratum. Intolerance is assessed as high. Recoverability is likely to be moderate (see Additional Information section below). | High | Moderate | Moderate | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceTBT and PCBs are generally considered to be toxic to marine invertebrates (Cole et al., 1999). However, there is insufficient information with respect to effects on Clavelina lepadiformis. | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceSeveral species of tunicate are known to accumulate high concentrations of trace metals. De Caralt et al. (2002) studied differences in certain aspects of the biology of Clavelina lepadiformis between harbour and open rocky littoral populations in the Mediterranean. They reported that Clavelina lepadiformis accumulated copper, lead and vanadium (vanadium is used in ascidian metabolism). The harbour population contained significantly more copper and lead than open littoral population despite its abundance being an order of magnitude higher in the harbour, suggesting that both adults and larvae are tolerant to this kind of contamination. Tolerant has been suggested. | Tolerant | Not relevant | Not sensitive | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceCole et al. (1999) stated that polycyclic aromatic hydrocarbons are of moderate toxicity to marine organisms. However there is insufficient information with regard to effects of hydrocarbons on Clavelina lepadiformis. | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficient Information. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceThere is some suggestion that there are possible benefits to the adults from increased organic content of water (Naranjo et al. 1996). | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceClavelina lepadiformis is tolerant of a wide range of salinities; Fish & Fish (1996) found that they could tolerate salinities as low as 14 psu. However, colonies in areas of full salinity are likely to be adversely affected by a short term acute change in salinity. Intolerance to salinity is assessed as low. Recoverability is likely to be immediate (see Additional Information below), when conditions return to normal or the sea squirt has adapted to the new levels. | Low | Immediate | Not sensitive | Low |
Decrease in salinity [Show more]Decrease in salinity
Evidence | No information | |||
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceCole et al. (1999) suggest possible adverse effects on marine species below 4 mg/l and probable adverse effects below 2 mg/l. No information was found concerning the tolerance of Clavelina lepadiformis to changes in oxygenation. However, the species lives in extremely sheltered conditions where oxygen depletion may occur. Therefore, intolerance has been assessed as low. Recoverability is likely to be immediate (see Additional Information below), once conditions return to normal or the species has adapted to the new oxygen concentration levels. | Low | Immediate | Not sensitive | Low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceInsufficient Information. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo alien or non-native species are known to affect Clavelina lepadiformis in Britain and Ireland. | Tolerant | Not relevant | Not sensitive | Low |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceIt is extremely unlikely that Clavelina lepadiformis will be subject to extraction. | Not relevant | Not relevant | Not relevant | Moderate |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceAdult Clavelina lepadiformis are not known to depend on other species. Therefore, the species is assessed as not sensitive. | Tolerant | Not relevant | Not sensitive | Moderate |
Additional information
Clavelina lepadiformis most likely has a short life span, of approximately 2 years. Each zooid reproduces once during June to September in temperate and cold seas (Picton & Costello, 1998; Millar, 1970) but in tropical waters it may continue throughout the year (Millar, 1970). In the Mediterranean, the breeding season is winter/spring (X. Turon, pers. comm.). Brunetti (1987) recorded up to about 50 embryos present in the atrium at one time. The larval phase is short, and metamorphosis into adults is rapid, so dispersal may be limited. Recolonization following incident is likely to be within a year providing that other nearby populations have survived. Rafting by adults attached to floating objects or shipping may form an important mechanism for recolonization.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | Not relevant |
Importance information
Clavelina lepadiformis is dominant over other organisms (Teo & Ryland, 1994).Bibliography
Bakus, G.J., 1968. Sedimentation and benthic invertebrates of Fanning Island, Central Pacific. Marine Geology, 6, 45-51.
Berrill, N.J., 1950. The Tunicata with an account of the British species. London: Ray Society.
Berrill, N.J., 1975. Chordata: Tunicata. In Reproduction of marine Invertebrates, vol. II, (ed. A.C. Geise & J.S. Pearse), pp. 241-282. New York: Academic Press.
Brunetti, R., 1987. Species of Clavelina in the Mediterranean Sea. Annales de l'Institut Oceanographique, Paris, 63, 101-118.
De Caralt, S., López-Legentil, S., Tarjuelo, I., Uriz, M.J. & Turon, X., 2002. Contrasting biological traits of Clavelina lepadiformis (Ascidiacea) populations from inside and outside harbours in the western Mediterranean. Marine Ecology Progress Series, 244, 125-137.
Fiana-Medioni, A., 1978. A scanning electron microscope study of the branchial sac of benthic filter-feeding invertebrates (ascidians). Acta Zoologica, 59, 1, 1-9.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Knight-Jones, E.W. & Ryland, J.S., 1995. Acorn-worms and sea squirts. In Handbook of the marine fauna of north-west Europe (ed. P.J. Hayward & J.S. Ryland), pp. 687-712. New York: Oxford University Press.
Millar, R.H., 1970. British Ascidians London: Academic Press.[Synopses of the British Fauna, no. 1.]
Moore, P.G., 1977a. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanography and Marine Biology: An Annual Review, 15, 225-363.
Naranjo, S.A., Carballo, J.L., & Garcia-Gomez, J.C., 1996. Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain). Possible marine bioindicators? Marine Ecology Progress Series, 144 (1), 119-131.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Steffan, B., 1991. Lepadin A, a decahydroquinoline alkaloid from the tunicate Clavelina & lepadiformis. Tetrahedron, 47, 8729-8732.
Tarjuelo, I. & Turon, X., 2004. Resource allocation in ascidians: reproductive investment vs. other life-history traits. Invertebrate Biology, 123, 168-180.
Tarjuelo, I., López-Legentil, S., Codina, M. & Turon, X., 2002. Defence mechanisms of adults and larvae of colonial ascidians: patterns of palatability and toxicity. Marine Ecology Progress Series, 235, 103-115.
Teo, S.L.-M. & Ryland, J.S., 1994. Toxicity and palatability of some British ascidians. Marine Biology, 120, 2, 297-303.
Teo, S.L.-M. & Ryland, J.S., 1995. Potential antifouling mechanisms using toxic chemicals in some British ascidians. Journal of Experimental Marine Biology and Ecology, 188, 49-62.
Turon, X., Tarjuelo, I., Duran, S. & Pascual, M., 2003. Characterising invasion processes with genetic data: and Atlantic clade of Clavelina lepadiformis (Ascidiacea) introduced into Mediterranean harbours. Hydrobiologia, 503, 29-35.
Tursi, A., Matarrese, A. & Scalera Liaci, L., 1977. Settling phenomena in Clavelina lepadiformis (Müller) (Tunicata). Oebalia, 3, 3-16.
Whittingham, D.G., 1967. Light-induction of shedding of gametes in Ciona intestinalis and Morgula manhattensis. Biological Bulletin, Marine Biological Laboratory, Woods Hole, 132, 292-298.
Datasets
Centre for Environmental Data and Recording, 2018. IBIS Project Data. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2024. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2024-11-20
South East Wales Biodiversity Records Centre, 2023. SEWBReC Marine and other Aquatic Invertebrates (South East Wales). Occurrence dataset:https://doi.org/10.15468/zxy1n6 accessed via GBIF.org on 2024-09-27.
Citation
This review can be cited as:
Last Updated: 02/09/2008