Ponto-Caspian freshwater hydroid (Cordylophora caspia)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters & Paolo Pizzolla | Refereed by | This information is not refereed |
| Authority | (Pallas, 1771) | ||
| Other common names | Freshwater hydroid | Synonyms | Cordylophora lacustris (Pallas, 1771) |
Summary
Description
A tall erect colony growing up to 10 cm high, branching occasionally from alternate sides and light horn to light brown in colour. Each branch is ringed at the base and has a terminal polyp. The polyps are white to pale pink and have 12 -16 long, colourless, extensile tentacles arranged irregularly on the surface of the polyp. The mouth is born on a conical but truncated proboscis. Each branch bears one to three pear-shaped reproductive bodies on short stalks. It produces a planula larvae but no free-living medusoid stage.
Recorded distribution in Britain and Ireland
The species has a sporadic distribution associated with areas of low salinity within estuaries and brackish lagoons.
Global distribution
Found in estuarine, lagoonal and coastal lake habitats in boreal to subtropical waters.
Habitat
This hydroid colonizes brackish waters of 2 -12 psu but, where salinity may rise, occasionally up to 35 psu. It is found in shallow depths, often in shade, on various hard substrata, submerged vegetation, and the shells of crabs and snails.
Depth range
Low shore to ca 2m.Identifying features
- Tall, erect colony ensheathed in perisarc.
- Polyps terminal with 12-16 tentacles.
- Polyps naked (athecate).
- Reproductive polyps (gonopores) pear-shaped.
Additional information
No text entered
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Cnidaria | Sea anemones, corals, sea firs & jellyfish |
| Class | Hydrozoa | White weeds, sea firs, sea beard and siphonophores; hydroids |
| Order | Anthoathecata | |
| Family | Cordylophoridae | |
| Genus | Cordylophora | |
| Authority | (Pallas, 1771) | |
| Recent Synonyms | Cordylophora lacustris (Pallas, 1771) | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Small-medium(3-10cm) | ||
| Female size at maturity | |||
| Growth form | Turf | ||
| Growth rate | ca 0.05-0.1mm/hr | ||
| Body flexibility | High (greater than 45 degrees) | ||
| Mobility | |||
| Characteristic feeding method | Passive suspension feeder, Predator, See additional information | ||
| Diet/food source | |||
| Typically feeds on | Small zooplankton, small crustacea, oligochaetes, insect larvae and probably detritus. | ||
| Sociability | |||
| Environmental position | Epibenthic | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No | ||
Biology information
Growth form
Growth form is highly variable in Cordylophora caspia. The colony of consists of a mass of stolons (hydrorhizae) growing across the surface of the substratum. Growth is apical, with side stolons arising at right angles. Upright hydrocauli bear apical polyps (hydranths), and side branches at 45° (Fulton, 1961).The degree of branching, length and spacing of hydrocauli, cell size, size and shape of polyps and the number and length of tentacles vary with environmental conditions (Fulton, 1962; Kinne, 1970, 1971; Arndt, 1989). For example, colonies have short, polyps that grow directly from the hydrorhiza at 0.5psu; more elongate polyps with longer tentacles and multiply branched uprights at 15psu, but smaller polyps and less branched uprights at 30psu than at 15psu (see Kinne 1970, 1971 for review; Arndt, 1989, Gili & Hughes, 1995). Gaulin et al. (1986) noted that predation by the nudibranch Tenellia fuscata resulted in denser colonies.
Growth rates
Growth rates are variable depending on environmental or laboratory conditions. Growth in number of polyps is exponential, the colonies doubling in polyp number every 2-4 days, although growth rate can very as much as two-fold even under standard conditions (Fulton, 1961, 1962). In addition, although old colonies could reach as much as 2000 polyps in size growth rates decreased with age (Fulton, 1962). Fulton (1961) reported that uprights grew at 0.05mm/hr while stolons extension rates vary from 0.1mm/hr (Fulton, 1961) to 2-3mm3/day (Chester et al., 2000). Fulton (1962) reported that growth rates varied with temperature, salinity, ionic composition, oxygen tension and feeding rate (see sensitivity).
Seasonal changes
Cordylophora caspia dies back in late autumn and overwinters as dormant stolons and resting stages (menonts) inside the remnants of the uprights (see Roos, 1979 for figure; Arndt, 1989; Jormalainen et al., 1994). Arndt (1989) reported that colonies died back in autumn when the temperature fell to about 10°C only to germinate in spring when the temperature exceeded 5°C. Roos (1979) reported that colonies died back in October and new polyps budded again in early spring in the Netherlands. In the Baltic Sea growth was maximal in spring, uprights reaching maximal height at the peak of sexual reproduction in July, with a decline after sexual reproduction, and subsequent growth in August (Jormalainen et al., 1994). However, in one year, Jormalainen et al (1994) noted that the colonies regressed to the dormant condition after sexual reproduction then started growing again by mid August.
Feeding
Hydroids are passive carnivores that capture prey that swim into, or are brought into contact with their tentacles by currents. Prey are then killed or stunned by the nematocysts born on the tentacles and swallowed. Diet varies but is likely to include small zooplankton (e.g. nauplii, copepods), small crustacea, chironomid larvae, detritus and oligochaetes, but may include a wide variety of other organisms such as the larvae or small adults of numerous groups (see Gili & Hughes, 1995).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Sea loch or Sea lough, Ria or Voe, Estuary, Isolated saline water (Lagoon), Enclosed coast or Embayment |
| Biological zone preferences | Lower eulittoral, Lower infralittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Caves, Cobbles, Large to very large boulders, Overhangs, Pebbles, Small boulders, Under boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Very sheltered |
| Salinity preferences | Low (<18 psu), Reduced (18-30 psu) |
| Depth range | Low shore to ca 2m. |
| Other preferences | No text entered |
| Migration Pattern |
Habitat Information
Habitat preferences
The distribution of Cordylophora caspia is determined by availability of suitable hard substratum, food availability, range and variability of temperature and salinity. Cordylophora caspia can survive between -10 °C (as resistant dormant stages, menonts) and 35 °C. Colonies tolerate 5 to 35 °C, and reproduce between 10 to 28 °C. It can also survive 0 to 35psu as resistant stages, grow between 0.2 to 30 psu, reproduce between 0.2 to 20psu and possesses the ability to ionic regulate (Kinne, 1971; reviewed by Arndt, 1986, 1989). In nature, well developed colonies are usually found in water of 2 -12psu where tidal influence is considerable or between 2 -6psu where conditions are constant (Arndt, 1989). It may also occur at full salinities, and fast flowing, well oxygenated freshwater containing Ca, Mg, Na Cl and K ions (Fulton, 1962; Arndt, 1989). Arndt (1986, 1989) suggested that respiration, growth and reproduction were optimal between 4-7psu and that food intake was high in comparison to other hydroids so that growth and reproduction rates required for the survival of the species could only occur in eutrophic or hypertrophic waters where food is plentiful. Its marine distribution is probably limited by food availability, competition from Clava spp. or Laomedea spp. and predation e.g. from the nudibranch Tenellia adspersa (as Embletonia pallida) (Arndt, 1989). Cordylophora caspia prefers conditions of low light (Allman, 1871-1872, Arndt, 1989), although light intensity did not affect growth (Fulton, 1963), which probably reflects the settlement preferences of the planula larvae.
Substrata
Most hydroids do not show a high specificity of substrata. Cordylophora caspia has been recorded from a wide variety of hard substrata including rocks, shells and artificial substrata (pilings, harbour installations, bridge supports), floating debris and occasionally from the leaves of reeds (Phragmites) or stalks of water lilies (MBA, 1957; Roos, 1979; Morri & Boero, 1986; Arndt, 1986, 1989; JNCC, 1999; Foster-Smith, 2000).
Non-native status
Cordylophora caspia was thought to have been introduced to British waters on foreign timber (Allman, 1871-1872). Cordylophora caspia was introduced into the Baltic Sea in ca 1803 and was reported as an alien species in the Baltic Sea and the Chesapeake Bay region, USA (Folino, 1999 (summary only); Olenin et al., 2000). Folino (1999, summary only) suggested that the distribution of Cordylophora spp. was expanding globally due to increased boat travel and ballast discharge.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Vegetative |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | See additional information |
| Generation time | <1 year |
| Age at maturity | Less than 1 month. |
| Season | Spring - Autumn |
| Life span | See additional information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Direct development |
| Duration of larval stage | < 1 day |
| Larval dispersal potential | <10 m |
| Larval settlement period | See additional information |
Life history information
Most hydroids (including Cordylophora caspia) are dioecious. The reproductive organs are carried in gonophores. Sperm are released into the sea and eggs are fertilized within the female gonophores where the embryos develop into planulae. Sperm swim towards female gonophores, however, sperm probably have a limited lifespan and hence a limited range for fertilization of only a few metres. Hence the growth of hydroids in clumps may enhance fertilization rates, albeit at the cost of intraspecific competition. Temperature is critical for stimulating or preventing reproduction in hydroids (see distribution; Gili & Hughes, 1995).Sexual reproduction
Early seasonal growth from winter dormancy in early spring is rapidly followed by formation of gonophores and sexual reproduction in midsummer followed by active growth in late summer. However, sexual reproductive effort may retard growth (see general biology). Jormalainen et al. (1994) reported that reproduction began in early June, peaked in July (80% uprights with gonophores) and rapidly reduced by August (30% uprights with gonophores). Similar reproductive periods have been reported by other authors (Allman, 1871-1872; MBA, 1957; Roos, 1979; Foster-Smith, 2000). Roos (1979) and Jormalainen et al. (1994) reported that the sex ratio was biased in favour of females.
Each upright branch may bear between 1-3 gonophores each with between 10 - 6 eggs, the number decreasing in autumn (Hincks, 1868; Jormalainen et al., 1994). Therefore, fecundity is dependant on the number of branches and hence the number of gonophores, and in large colonies of 70-2000 polyps (Fulton, 1962), may be high. The larvae are released as planulae and no medusoid stage occurs. However, in some cases the larvae may develop directly into juvenile polyps in the gonophore before release (Bouillon, 1963).
Asexual reproduction
Hydroids may reproduce asexually by budding to from another colony. A common form of asexual reproduction in hydroids is the formation of vertical stolons, which then adhere to adjacent substratum, detach and form another colony (Gili & Hughes, 1995). Hydroids exhibit remarkable powers of regeneration and Cordylophora caspia can be cloned in culture from detached uprights or excised tissue (Moore, 1952;Fulton, 1961, 1962). Asexual reproduction by fission or mechanical fragmentation of the colony may be an important factor in dispersal (Gili & Hughes, 1995).
Longevity
While uprights have a short, finite lifespan from about early spring to autumn, no information concerning the lifespan of the dormant stages (menonts) was found. Unless destroyed by predators or physical damage, the colony may have a long lifespan (perhaps very long (Gili & Hughes, 1995). The ability to reproduce asexually and regenerate from damaged sections means that although any individual colony may have a finite lifespan the genetic individual (genet) may be considerably longer lived (Gili & Hughes, 1995).
Dispersal
Rapid growth, budding and the formation of stolons allows hydroids to colonize space rapidly. Hydroids are often the first organisms to colonize available space in settlement experiments (Gili & Hughes, 1995). Planula larvae swim or crawl for short periods (e.g. <24hrs) so that dispersal away from the parent colony is probably very limited (Gili & Hughes, 1995). Fragmentation may also provide another route for short distance dispersal. However, it has been suggested that rafting on floating debris (or hitch hiking on ships hulls or in ship ballast water) as dormant stages or reproductive adults, together with their potentially long lifespan, may have allowed hydroids to disperse over a wide area in the long-term and explain the near cosmopolitan distributions of many hydroid species, including Cordylophora caspia (Gili & Hughes, 1995; Folino, 1999).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceRemoval of the substratum would result in the loss of colonies, their hydrorhizae and any resting stages. Therefore, an intolerance of high has been recorded. Recovery will depend on whether nearby colonies or colonies within the same water body have survived. If so, recoverability will be rapid, although the original population abundance may take several growing seasons to achieve, and an intolerance of high has been recorded. If the population has been completely destroyed recoverability will be low or not at all (see additional information below). | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceHydroids usually colonize overhanging, vertical or steeply sloping surfaces presumably to avoid the possibility of siltation and smothering and competition from macroalgae. Smothering by 5cm of sediment (see benchmark) is likely to cover a large proportion of the colony, preventing feeding and hence reducing growth and reproduction. In addition, local hypoxic conditions are also likely to inhibit growth (Fulton, 1961, 1963). However, the colony is likely to become dormant, or otherwise survive for a period of at least a month, and recover rapidly once the sediment is removed. Therefore, an intolerance of low has been recorded to represent the affects of smothering on growth.Recovery is likely to be immediate (see additional information below). | Low | Immediate | Not sensitive | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceCordylophora caspia is found in estuarine and sheltered lagoonal habitats, which are characterized by high suspended sediment loads. Cordylophora caspia was also reported in saltmarsh pools (JNCC, 1999) and saltmarshes are a depositional environment characterized by siltation. Therefore, Cordylophora caspia is probably tolerant of increases in suspended sediment loads at the benchmark level. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceA reduction in suspended sediment is unlikely to directly affect Cordylophora caspia. A decrease in suspended sediment may also reduce the availability of organic particulates and hence reduce food availability. Arndt (1986, 1989) suggested that Cordylophora caspia had a high food requirement for growth and reproduction. It is therefore, likely to be intolerant of any reduction in food availability and an intolerance of low has been recorded. Recovery is likely to be immediate. | Low | Immediate | Not sensitive | Low |
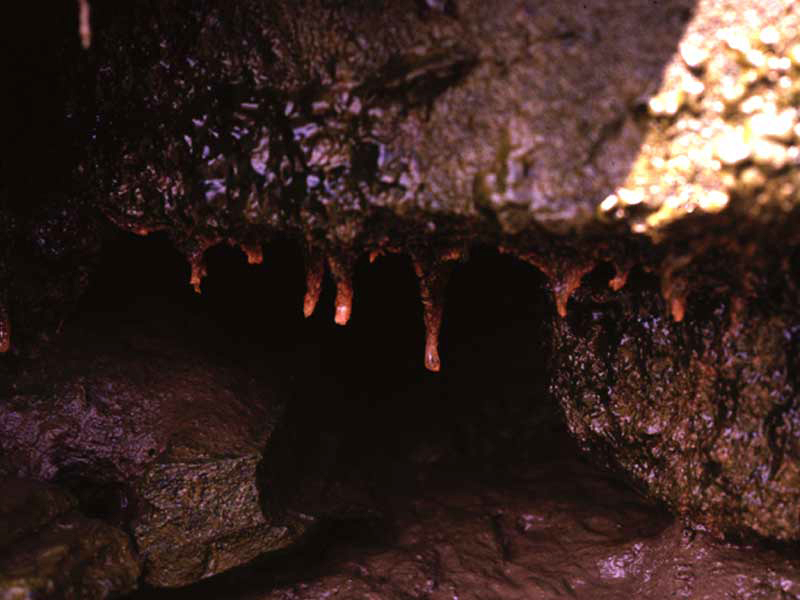
Desiccation [Show more]Desiccation
EvidenceIntertidal populations of Cordylophora caspia are restricted to damp habitats such as underboulders and overhangs. The branched growth form of this species is likely to retain water on emersion (see image). However, an increase in desiccation at the benchmark level is likely to result in drying and death of the uprights. Increased desiccation may result in the formation of resistant, dormant stages, however, no information on their desiccation tolerance was found. Therefore, an intolerance of high has been recorded.If hydrorhizae or dormant stages survive recovery is likely to be very rapid and colonies may appear rapidly once conditions return to their prior state. If, resting stages are destroyed then recovery will depend on recruitment from nearby subtidal colonies, and is likely to be rapid, although the original population abundance may take several growing seasons to achieve (see additional information below). | High | High | Moderate | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAn increase in emergence is likely to adversely affect colonies. While Cordylophora caspia would probably survive the extremes of temperature resulting form increased emergence (see below), colonies are likely to succumb to increased desiccation (see above) if increased emergence exposes them to mid shore (or higher) conditions. Therefore, the upper shore proportion of the population is likely to be lost and an intolerance of intermediate has been recorded. Recoverability is likely to be very high (see additional information below). | Intermediate | Very high | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA decrease in emergence is likely to increase the availability of suitable habitats and may allow the population to extend its range. Therefore, tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceWater movement is essential for hydroids to supply adequate food, remove metabolic waste products, prevent accumulation of sediment and disperse larvae or medusae. Hydroids are expected to be abundant where water movement is sufficient to supply adequate food but not cause damage (Hiscock, 1983; Gili & Hughes, 1995). Flexibility of the otherwise rigid perisarc of hydroids is provided by annulations at the base of branches in Cordylophora caspia. In addition, in athecates, the neck of the polyp is flexible enough to allow the polyp adopt an efficient 'lee-side' feeding posture in water flow. However, most hydroids have a narrow range of water flow rates for effective feeding. For example in the athecate Tubularia indivisa, food capture rate increased up to 20cm/s, but decreased as water flow rates increased (Hiscock, 1983). In Cordylophora inkermania food capture rates were higher in fluctuating flows than in unidirectional flows (Gili & Hughes, 1995), presumably because more polyps were brought into play in fluctuating flow, than in unidirectional flow where upstream branches 'shaded' down stream branches. Loomis (in Fulton, 1961) noted that Cordylophora caspia did not grow in still water cultures presumably because of the build up of CO2 from respiration. Cordylophora caspia was reported to dominate steep rock surfaces in strong tidal flows in the Tamar (JNCC, 1999). Although Cordylophora caspia tolerates strong water flow, a further increase in water flow to very strong is likely to reduce feeding efficiency and hence growth and reproduction and may even remove or damage colonies. Damaged colonies may survive as resting stages until water flow rates return to prior condition. Therefore, intolerance has been assessed as low and recoverability as very high (see additional information below). | Low | Very high | Very Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceWater movement is essential for hydroids to supply adequate food, remove metabolic waste products, prevent accumulation of sediment and disperse larvae or medusae (see above). Cordylophora caspia has been recorded from areas of negligible or weak water flow, e.g. saline lagoons. A further decrease in water flow is unlikely, although Loomis (in Fulton, 1961) noted that colonies did not grow in still water cultures presumably because of the build up of CO2 from respiration. Therefore, an intolerance of low has been recorded. Recovery is likely to be very high (see additional information below). | Low | Immediate | Not sensitive | Low |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceCordylophora caspia can survive as resistant dormant stages (menonts) at -10 °C and at 35 °C. Colonies tolerate 5 to 35 °C, and reproduce between 10 to 28 °C. Arndt (1989) reported that colonies died back in autumn when the temperature fell to about 10 °C only to germinate in spring when the temperature exceeded 5 °C. Arndt (1989) concluded that Cordylophora caspia was thermophilic but that low temperature had an important influence on growth and reproduction. In addition, the distribution of Cordylophora caspia extends into subtropical habitats (Arndt, 1986, 1989). Therefore, this species is unlikely to be adversely affected by chronic or acute temperature change at the benchmark level in British waters. | Tolerant | Not relevant | Not sensitive | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceCordylophora caspia can survive as resistant dormant stages (menonts) at -10 °C and at 35 °C. Colonies tolerate 5 to 35 °C, and reproduce between 10 to 28 °C. Arndt (1989) reported that colonies died back in autumn when the temperature fell to about 10 °C only to germinate in spring when the temperature exceeded 5 °C. Arndt (1989) concluded that Cordylophora caspia was thermophilic but that low temperature had an important influence on growth and reproduction. Therefore, while low temperatures may trigger premature die back or regression, colonies are likely to survive changes in temperature at the benchmark level and an intolerance of low has been recorded. | Low | Very high | Very Low | Moderate |
Increase in turbidity [Show more]Increase in turbidity
EvidenceCordylophora caspia is unlikely to be directly influenced by light intensity (Fulton, 1962). Cordylophora caspia and other hydroids tend to shun well lit conditions, planulae becoming negatively phototactic prior to settlement, presumably to avoid competition with macroalgae (Gili & Hughes, 1995). Therefore, a decrease in light penetration may decrease competition for space with macroalgae. However, decrease light penetration may also decrease phytoplankton and hence zooplankton productivity and potentially decrease food availability, and an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceIncreased light penetration may increase phytoplankton and hence zooplankton productivity and potentially increase food availability. Therefore, tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceThe oscillatory water flow caused by wave action is potentially more damaging to delicate marine organisms than unidirectional flow. Although, the annuli at the base of branches gives the branched colony of Cordylophora caspia some flexibility it has only been recorded from very or extremely wave sheltered habitats (JNCC, 1999). Therefore, it is likely than an increase in wave exposure at the benchmark level (e.g. from 'very sheltered' to 'moderately exposed') is likely to result in loss of the colonies. Populations occupying small rocks, cobbles or pebbles are likely to be more intolerant, and the resultant movement of the substratum and sediment scour may also remove attached hydrorhizae and even resting stages. Therefore, an intolerance of high has been recorded.Recovery will depend in part on recruitment from other areas. However, any resting stages and fragments of colonies remaining may contribute to the recovery, although the original population abundance may take several growing seasons to achieve. Therefore, a recoverability of high has been suggested. | High | High | Moderate | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceCordylophora caspia has only been recorded from very or extremely wave sheltered habitats (JNCC, 1999). A decrease in wave exposure may allow the species to colonize additional habitats and increase its extent. Therefore, tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Not relevant |
Noise [Show more]Noise
EvidenceHydroids are unlikely to be sensitive to noise or vibration at the benchmark level. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceHydroid polyps may retract when shaded by potential predators, however hydroids are unlikely to be affected by visual presence. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion by an anchor or fishing gear is likely to remove relatively delicate upright parts of the colony. However, the surface covering of hydrorhizae may remain largely intact, from which new uprights are likely to grow. In addition, the resultant fragments of colonies may be able to develop into new colonies (see displacement). Populations on small hard substrata (e.g. cobbles, pebbles or stones) may be removed by fishing gear, constituting substratum loss (see above). Overall, a proportion of the colonies is likely to be destroyed and an intolerance of intermediate has been recorded. However, recovery from surviving hydrorhizae and occasional fragments is likely to be rapid (see additional information below). | Intermediate | Very high | Low | Low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceCordylophora caspia colonies have been cultured by securing cut uprights to slides to which they subsequently attach. Fragmentation is thought to be a possible mode of asexual reproduction in hydroids (Gili & Hughes, 1995). Therefore, it is possible that a proportion of displaced colonies (or fragments thereof) may attach to new substrata and an intolerance of intermediate has been recorded. Recovery is likely to be rapid (see additional information below). | Intermediate | Very high | Low | Low |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceThe species richness of hydroid communities decreases with increasing pollution but hydroid species adapted to a wide variation in environmental factors and with cosmopolitan distributions tend to be more tolerant of polluted waters (Boero, 1984; Gili & Hughes, 1995). Stebbing (1981) reported that Cu, Cd, and tributyl tin fluoride affected growth regulators in Laomedea (as Campanularia) flexuosa resulting in increased growth. Bryan & Gibbs (1991) reported that virtually no hydroids were present on hard bottom communities in TBT contaminated sites and suggested that some hydroids were sensitive to TBT levels between 100 and 500 ng/l.However, Calder (1976) suggested that hydroids found in the low salinity areas of south Carolina, such as Cordylophora caspia, were also present in relatively polluted waters, such as Charleston Harbour. Cordylophora caspia was also a dominant species on settlement plates placed on a floating shipyard dock in Warnock river (Sandrock et al., 1991). Floating docks are likely to result in local contamination with heavy metals and antifouling agents from ship paints, as well as oils and other chemicals used in ship maintenance. As a member of fouling communities, Cordylophora caspia is probably less intolerant of antifouling measures than other hydroids. Therefore, an intolerance of low has been suggested albeit at very low confidence. | Low | Immediate | Not sensitive | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceStebbing (1981) reported that Cu, Cd, and tributyl tin fluoride affected growth regulators in Laomedea (as Campanularia) flexuosa resulting in increased growth. Ringelband (2001), however, reported that 1.74-7.96 mg/l vanadium inhibited growth of Cordylophora caspia at low salinities. Various heavy metals have also been show to have sublethal effects on growth in the few hydroids studied experimentally (Bryan, 1984). Therefore, an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceLittle information of the effects of hydrocarbons on hydroids was found. Hydroid species adapted to a wide variation in environmental factors and with cosmopolitan distributions tend to be more tolerant of polluted waters (Boero, 1984; Gili & Hughes, 1995). Calder (1976) suggested that hydroids found in the low salinity areas of south Carolina, such as Cordylophora caspia, were also present in relatively polluted waters, such as Charleston Harbour. Cordylophora caspia was also a dominant species on settlement plates placed on a floating shipyard dock in Warnock river (Sandrock et al., 1991). Floating docks are likely to result in local contamination with heavy metals and antifouling agents from ship paints, as well as oils and other chemicals used in ship maintenance. However, the above evidence is primarily anecdotal.The water soluble fractions of Monterey crude oil and drilling muds were reported to cause polyp shedding and other sublethal effects in the athecate Tubularia crocea in laboratory tests (Michel & Case, 1984; Michel et al., 1986; Holt et al., 1995). The athecate Cordylophora caspia may show similar sublethal effects assuming similar physiology. Therefore, an intolerance of low has been recorded albeit with a very low confidence. | Low | Immediate | Not sensitive | Very low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceCordylophora caspia became one of the dominant species to colonize settlement plates placed beneath a floating dock in the Warnock river (Sandock et al., 1991). This station was characterized by low salinities, and higher organic and mineral nutrient loads (ca 20-100 µmol NO3/l) than their other experimental station. Arndt (1986, 1989) suggested that food intake in Cordylophora caspia was high in comparison to other hydroids so that growth and reproduction rates required for the survival of the species could only occur in eutrophic or hypertrophic waters where food is plentiful. Therefore, Cordylophora caspia is likely to tolerate relatively high nutrient levels, and may benefit from moderate increases in nutrients at the benchmark level. Hence, tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Increase in salinity [Show more]Increase in salinity
EvidenceCordylophora caspia can survive 0 to 35psu as resistant stages, grow between 0.2-30 psu, reproduce between 0.2-20psu and possesses the ability to ionic regulate (Kinne, 1971; reviewed by Arndt, 1986, 1989). In nature, well developed colonies are usually found in water of 2 -12psu where tidal influence is considerable or between 2 -6psu where conditions are constant (Arndt, 1989) but it may also occur at full salinities. Kinne (1971) noted that high salinities (24 or 30psu) occasionally resulting in developmental abnormalities in older colonies in the laboratory.Arndt (1989) suggested that its marine distribution was probably limited by food availability, competition from Clava spp. or Laomedea spp. and predation e.g. from the nudibranch Tenellia adspersa (as Embletonia pallida). Therefore a short term increase in salinity (at the benchmark level) is likely to affect growth and reproduction but otherwise not adversely affect colonies. However, a change in salinity from reduced to variable in the long term (see benchmark) may result in loss of a proportion of the population, and an intolerance of intermediate has been recorded. Survival of resting stages is likely to result in rapid recovery (see additional information below). | Intermediate | Very high | Low | Moderate |
Decrease in salinity [Show more]Decrease in salinity
EvidenceCordylophora caspia can survive 0 to 35psu as resistant stages, grow between 0.2-30 psu, reproduce between 0.2-20psu and possesses the ability to ionic regulate (Kinne, 1971; reviewed by Arndt, 1986, 1989). In nature, well developed colonies are usually found in water of 2 -12psu where tidal influence is considerable or between 2 -6psu where conditions are constant (Arndt, 1989). It may also occur at full salinities, and fast flowing, well oxygenated freshwater containing Ca, Mg, Na, Cl and K ions (Fulton, 1962; Arndt, 1989). It has been reported from estuaries that receive significant seasonal freshwater input, and tolerates variable salinities (Arndt, 1986; 1989). Therefore, it is probably relatively tolerant of a change in salinity at the benchmark level. A reduction from full to reduced salinity may be beneficial and allow Cordylophora caspia to colonize new habitats. Therefore, not sensitive* has been recorded. | Tolerant* | Not relevant | Not sensitive* | High |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceFulton (1962) found that some polyps of Cordylophora caspia fell off or were reabsorbed after 7 days in the complete absence of oxygen, however, the remaining polyps began feeding shortly after the re-introduction of oxygen. Fulton (1962) concluded that Cordylophora caspia had a low oxygen requirement for growth and was able to grow at oxygen levels of >2mg/l (ca 1.4ml/l). Arndt (1986) reported that an increase in temperature from 10 to 20 °C resulted in a marked increase in metabolic rate and hence oxygen consumption. Similarly, metabolic rates increased at supra- or subnormal salinities (Arndt, 1986) and Cordylophora caspia may be more intolerant of low oxygen concentrations at high temperatures and extreme salinities. However, the isolated saline waters, the upper reaches of estuaries and saltmarsh pools, in which Cordylophora caspia occurs, are likely to experience high summer temperatures and hence low oxygen levels. Therefore, Cordylophora caspia is likely to survive exposure to low oxygen concentrations at the benchmark level, although growth is likely to be reduced, and an intolerance of low has been recorded. | Low | Immediate | Not sensitive | High |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceCordylophora caspia is a non-native species (Allman, 1871-1872). But has not been reported to compete with other species, including other non-native species, in British or Irish waters. | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceHydroids are not known to be subject to specific extraction. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceCordylophora caspia is not known to be associated with species or habitats subject to extraction. | Not relevant | Not relevant | Not relevant | Not relevant |
Additional information
intolerance assessmentCordylophora caspia and other hydroids have the ability to produce dormant resting stages (menonts) that are far more resistant to environmental change than the colony itself. Therefore, although colonies may be removed or destroyed, the resting stages may survive in remnants of the hydrorhizae attached to the substratum. For the sake of assessment, the intolerance of the branched colonies themselves ( the clearly visible component) has been recorded. The resting stages provide a mechanism for rapid recovery.
Recoverability
Hydroids are often initial colonizing organisms in settlement experiments and fouling communities (Jensen et al., 1994; Hatcher, 1998). In settlement experiments in the Warnow estuary, Cordylophora caspia was found to colonize artificial substrata within ca one month of deployment, its abundance increasing from June to the end of September with a peak in July (Sandrock et al., 1991). Long term panels at the low salinity station became dominated by Cordylophora caspia, %Balanus improvisus% and %Nais elinguis%. Similarly, Roos (1979) reported that Cordylophora caspia recruited to and grew luxuriantly on water lily stalks in summer after early reproduction of nearby colonies in early spring. Therefore, it is likely that Cordylophora caspia will recruit to available space rapidly in its growing season, in the vicinity of other populations. Once colonized the hydroids ability to grow rapidly and reproduce asexually is likely to allow it to occupy space and sexually reproduce quickly, possibly recruiting to additional space before dying back in winter. Therefore, where colonies or dormant resting stages are present in the habitat, or within isolated habitats (e.g. lagoons), recovery is likely to be rapid and occur within less than a year.
Long distance dispersal is probably limited in hydroids, including Cordylophora caspia. Long distance dispersal is probably dependant on passive dispersal by currents on floating debris or shipping. Although, Cordylophora caspia has probably been introduced to the coasts of several countries (e.g. Chesapeake Bay USA and the Baltic Sea), passive transportation is a sporadic and un-predictable event. Therefore, if the whole population is destroyed, and resting stages removed, recolonization rates will depend on distance from nearby colonies, and may take many years. In isolated habitats such as lagoons and coastal lakes, recruitment from other areas may take a many decades or not occur at all.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | Non-native |
| Origin | East Europe, Soviet Middle Asia |
| Date Arrived | 1868 |
Importance information
Hydroids are preyed on by chitons, gastropods (especially nudibranchs), polychaetes and pycnogonids (Gili & Hughes, 1995). For example, the pycnogonid Anoplodactylus sp. was reported to feed on Cordylophora caspia in Australasia (Staples & Watson, 1987) and may do so in the British Isles.The life cycle of nudibranchs is often closely linked with the seasonal availability of their preferred prey. Arndt (1989) noted that the marine distribution of Cordylophora caspia was limited in part by predation by the nudibranch Tenellia adspersa (as Embletonia pallida). Gaulin et al. (1986) noted that a healthy colony would survive limited nudibranch predation by Tenellia fuscata but be removed by large numbers of predators. For example, Chester et al. (2000) suggested that Tenellia adspera showed rapid population growth, removing the hydroid and preventing its reestablishment. Nudibranch predation is probably an important factor limiting the presence of hydroids in early community succession, removing the hydroids and allowing colonization of other species.
Bibliography
Allman, G.J., 1871-1872. A monograph of the Gymnoblastic or Tubularian hydroids, Vol. I & II. London: Ray Society.
Arndt, E.A., 1984. The ecological niche of Cordylophora caspia (Pallas, 1771). Limnologica, 15, 469-477.
Arndt, E.A., 1989. Ecological, physiological and historical aspects of brackish water fauna distribution. In Proceedings of the 23rd European Marine Biology Symposium, Swansea, 5-9 September 1988. Reproduction, Genetics and Distribution of Marine Organisms, (ed. J.S. Ryland & P.A. Tyler), pp. 327-338. Denmark: Olsen & Olsen.
Barnes, R.S.K., 1994. The brackish-water fauna of northwestern Europe. Cambridge: Cambridge University Press.
Boero, F., 1984. The ecology of marine hydroids and effects of environmental factors: a review. Marine Ecology, 5, 93-118.
Bouillon, J., 1963. Sur quelques particularités de la reproduction sexuée de Cordylophora caspia (Pallas 1771). Annales de la Société Royale Zoologique de Belgique, 93, 157-158.
Bryan, G.W. & Gibbs, P.E., 1991. Impact of low concentrations of tributyltin (TBT) on marine organisms: a review. In: Metal ecotoxicology: concepts and applications (ed. M.C. Newman & A.W. McIntosh), pp. 323-361. Boston: Lewis Publishers Inc.
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Calder, D.R., 1976. The zonation of hydroid colonies along salinity gradients in South Carolina estuaries. In Coelenterate ecology and behaviour (ed. G.O. Mackie), pp. 165-174. New York: Plenum Press.
Campbell, R.D., 1974. Cnidaria. In Reproduction of marine invertebrates vol. I. Acoelomate and Pseudocoelomate metazoans (ed. A.C. Gieses & J.S. Pearse), pp. 133-199. New York: Academic Press.
Chester, C.M., Turner, R., Carle, M & Harris, L.G., 2000. Life history of a hydroid/nudibranch association: a discrete-event simulation. Veliger, 43, 338-348.
Folino, N.C., 1999. The freshwater expansion and classification of the colonial hydroid Cordylophora (Phylum Cnidaria, Class Hydrozoa). In Proceedings of the first National Conference, Massachusetts Institiute of Technology, Cambridge, MA, 1999. Marine Bioinvasions, pp. 139-144.
Foster-Smith, J. (ed.), 2000. The marine fauna and flora of the Cullercoats District. Marine species records for the North East Coast of England. Sunderland: Penshaw Press, for the Dove Marine Laboratory, University of Newcastle upon Tyne.
Fulton, C., 1961. The development of Cordylophora. In The biology of Hydra and some other coelenterates (ed. H.M. Lenholf & W.F. Loomis), pp. 287-295. Miami Florida: University of Miami Press.
Fulton, C., 1962. Environmental factors influencing the growth of Cordylophora. Journal of Experimental Zoology, 151, 61-78.
Gaulin, G., Dill, L., Beaulieu, J. & Harris, L.G., 1986. Predation-induced changes in growth form in a nudibranch-hydroid association. Veliger, 28, 389-393.
Gili, J-M. & Hughes, R.G., 1995. The ecology of marine benthic hydroids. Oceanography and Marine Biology: an Annual Review, 33, 351-426.
Hatcher, A.M., 1998. Epibenthic colonization patterns on slabs of stabilised coal-waste in Poole Bay, UK. Hydrobiologia, 367, 153-162.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hincks, T., 1868. A history of the British hydroid zoophytes, vol. I & II. London: van Voorst.
Jensen, A.C., Collins, K.J., Lockwood, A.P.M., Mallinson, J.J. & Turnpenny, W.H., 1994. Colonization and fishery potential of a coal-ash artificial reef, Poole Bay, United Kingdom. Bulletin of Marine Science, 55, 1263-1276.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Jormalainen, V., Honkanen, T., Vuorisalo, T. & Laihonen, P., 1994. Growth and reproduction of an estuarine population of the colonial hydroid Cordylophora caspia (Pallas) in the northern Baltic Sea. Helgoländer Meeresuntersuchungen, 48, 407-418.
Kinne, O. (ed.), 1970. Marine Ecology: A Comprehensive Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors Part 1. Chichester: John Wiley & Sons
Kinne, O. (ed.), 1971a. Marine Ecology: A Comprehensive, Integrated Treatise on Life in Oceans and Coastal Waters. Vol. 1 Environmental Factors, Part 2. Chichester: John Wiley & Sons.
MBA (Marine Biological Association), 1957. Plymouth Marine Fauna. Plymouth: Marine Biological Association of the United Kingdom.
Michel, W.C. & Case, J.F., 1984. Effects of a water-soluble petroleum fraction on the behaviour of the hydroid coelenterate Tubularia crocea. Marine Environmental Research, 13, 161-176.
Michel, W.C., Sanfilippo, K. & Case, J.F., 1986. Drilling mud evoked hydranth shedding in the hydroid Tubularia crocea. Marine Pollution Bulletin, 17, 415-419.
Morri, C. & Boero, F., 1986. Catalogue of main marine fouling organisms, vol. 7. Hydroids. Bruxelles: Office d'Etudes Marine et Atmosphériques.
Olenin, S., Daunys, D. & Dauniene, E., 2000. Baltic Sea alien species database. [On-line] http://www.ku.lt/nemo/mainnemo.htm, 2002-03-28
Ringelband, U., 2001. Salinity dependence of vanadium toxicity against the brackish water hydroid Cordylophora caspia. Ecotoxicology and Environmental Safety, 48, 18-26.
Roos, P.J., 1979. Two-stage life cycle of a Cordylophora population in the Netherlands. Hydrobiologia, 62, 231-239.
Sandrock, S., Scharf, E-M., von Oertzen, J.A., 1991. Short-term changes in settlement of micro- and macro-fouling organisms in brackish waters. Acta Ichthyologica et Piscatoria, 21(Suppl.), 221-235.
Sommer, C., 1992. Larval biology and dispersal of Eudendrium racemosum (Hydrozoa, Eudendriidae). Scientia Marina, 56, 205-211. [Proceedings of 2nd International Workshop of the Hydrozoan Society, Spain, September 1991. Aspects of hydrozoan biology (ed. J. Bouillon, F. Cicognia, J.M. Gili & R.G. Hughes).]
Staples, D.A. & Watson, J.E., 1987. Associations between pycnogonids and hydroids. In Modern trends in the systematics, ecology, and evolutions of hydroids and hydromedusae (ed. J. Bouillon, F. Boero, F. Cicogna, P.F.S. Cornelius), pp. 215-226. New York: Oxford University Press.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-02
Suffolk Biodiversity Information Service., 2017. Suffolk Biodiversity Information Service (SBIS) Dataset. Occurrence dataset: https://doi.org/10.15468/ab4vwo accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 03/07/2007