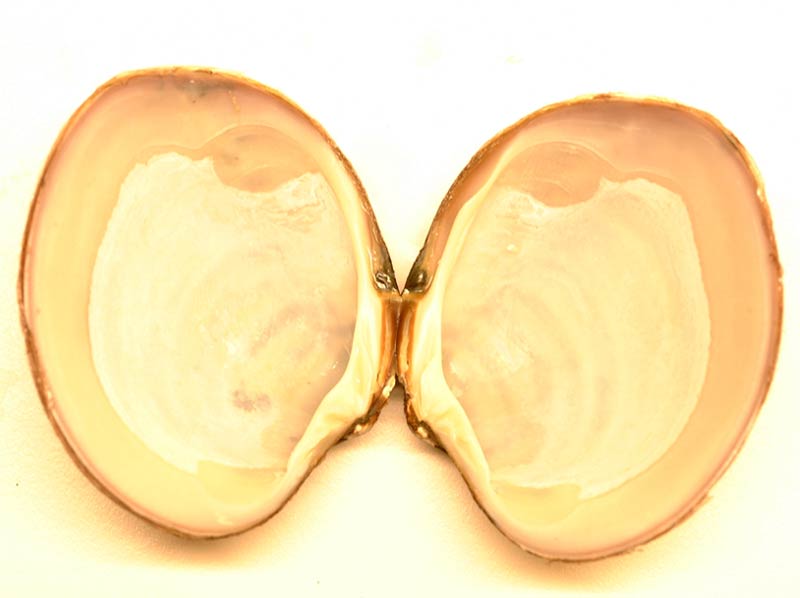
Basket shell (Varicorbula gibba)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Marisa Sabatini & Susie Ballerstedt | Refereed by | This information is not refereed |
| Authority | (Olivi, 1792) | ||
| Other common names | - | Synonyms | Corbula gibba (Olivi, 1792) |
Summary
Description
Varicorbula gibba has a plump, broadly oval to triangular shell up to 15 mm long. It is inequivalve, the right valve is very much larger and more convex than the left, which fits snugly into it, leaving a considerable margin of the right valve bare. The posterior margin is slightly truncate. Both valves of Varicorbula gibba are sculptured with coarse, concentric grooves and ridges, the left valve additionally having faint radiating lines. The beaks are turned inward and touching. The shell is dull white to cream, the interior is white with a faint pinkish or bluish tinge, sometimes with blotches of yellow. The pallial line is very faint and there is a slight posterior indentation or sinus.
Recorded distribution in Britain and Ireland
Common and widespread on all British coasts. The species is probably more widespread than mapped but records are not readily available.
Global distribution
Varicorbula gibba is distributed from the Norwegian Sea south to the Iberian Peninsula, into the Mediterranean and Black Seas, and along the coast of West Africa to Angola.
Habitat
Varicorbula gibba is found from the low shore to considerable depths in the sublittoral, living in muddy sand and gravel.
Depth range
ELW - 146 mIdentifying features
- The right valve is much larger and more convex than the left valve.
- The shell has a plump, broadly oval to triangular shape.
- The shell is up to 15 mm in length.
- Both valves are sculptured with coarse, concentric grooves and ridges, the left valve additionally having faint radiating lines.
- In front of the chondrophore of the left valve is a deep triangular pit into which a projecting cardinal tooth-like structure on the right valve fits.
Additional information
No text entered
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Bivalvia | Clams, cockles, mussels, oysters, and scallops |
| Order | Myida | Gapers, piddocks, and shipworms |
| Family | Corbulidae | |
| Genus | Varicorbula | |
| Authority | (Olivi, 1792) | |
| Recent Synonyms | Corbula gibba (Olivi, 1792) | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Small(1-2cm) | ||
| Female size at maturity | |||
| Growth form | Bivalved | ||
| Growth rate | See additional information | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Burrower | ||
| Characteristic feeding method | Active suspension feeder | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Phytoplankton, diatoms and bacteria. | ||
| Sociability | Solitary | ||
| Environmental position | Infaunal | ||
| Dependency | - | ||
| Supports | - | ||
| Is the species harmful? | No | ||
Biology information
Growth. The growth rate of Varicorbula gibba is rapid during the first few months of its juvenile stage although it is very slow in adults (Jensen, 1990). In Nissum Bredning, Denmark the growth of juvenile Varicorbula gibba was rapid during their first two months (July-August) but levelled off in September and October at lengths ranging from 2.9-3.5 mm (Jensen, 1988). Thus juvenile Varicorbula gibba reached a size of 3 mm within the first 1 to 2 months after settling (Jensen, 1990). The absolute growth rate for that period was about 0.03 mm/day and remained constant until the end of August. No further growth was observed in September and October (Jensen, 1988). One year after juvenile settlement, specimens reached a mean size of 6 -7 mm. In the Limfjord (Denmark) it was suggested that the variation in growth rates was caused by variable frequencies of wind-induced resuspension of settled organic matter. In the Limfjord wind speeds above Beaufort force 8 caused the mixing of the water column and probably the resuspension of bottom material in 1986. These conditions probably favour Varicorbula gibba as it is one of the most efficient bivalve particle feeders (Kiøboe & Mohlenberg, 1981). In 1985 the wind speeds never exceeded force 8 and no mixing was observed. This resulted in lower abundances of Varicorbula gibba and slower growth rates (Jensen, 1990).
Slower growth rates have been recorded in the Danish Sound where it took a population of Varicorbula gibba seven months to reach a mean size of 1.1 mm (Muss, 1973). Whereas in Port Erin on the Isle of Man, it took one year for a population of juveniles to reach a mean size of 4 mm (Jones, 1956, Jensen, 1990). Jones (1956) also reported that the specimens of Varicorbula gibba on the Isle of Man had a modal length of 2.25 mm. Jensen (1990) suggested that the higher growth rates in the 1990s in Danish waters could be the result of specific events such as eutrophication. However, in Nissum Bredning no specimens were found over two years old in 1990. The size of Varicorbula gibba around the British Isles ranged from 0.5 mm in length to 1.2 cm in the 1940s (Yonge, 1946), and in Australian waters, it can reach sizes up to 1.5 cm (CRIMP, 2000).
Abundance. Varicorbula gibba is often found in very large numbers and is often abundant in eutrophic areas (Pearson & Rosenberg, 1978). Varicorbula gibba are known to occur in enormous numbers, for instance, 7450 /m² at certain localities in the Atlantic (Healy & Lamprell, 1996). In the Limfjord, sampling of Varicorbula gibba was carried out at monthly intervals from April 1986 to May 1988. Ten samples were taken with a HAPS-corer (0.014 m²) and sieved over a 1 mm sieve (Jensen, 1990). The density for Varicorbula gibba ranged from 9,000 to around 53,000 per m². Newly settled Varicorbula gibba ranged from 30,000 and 67,000 individuals per m² (Jensen, 1988). In Pula Harbour in the Northern Adriatic, Varicorbula gibba was found at densities ranging from 33 -121 individuals / 0.2 m² (Hrs-Brenko, 1981). Varicorbula gibba is also found in Australia, outside of its native range, at densities of up to 250/m² in Port Philip Bay (CRIMP, 2000).
Biomass and production. During 1974-1984 nitrogen concentration and primary production of specimens of Varicorbula gibba in Nissum Bredning increased from 50-100% and 200-300%. The production (P) of Varicorbula gibba is generally high. Productivity was measured over two years and ranged from 0.7-72 g AFDW (ash free dry weight) m²/ yr. with an average of 26.8g AFDW m² / yr. in Nissum Bredning. The production / biomass ratio was amongst the highest recorded with a mean P / B of 4:2 per year (Jensen, 1990).
Respiration. Laboratory studies have shown that Varicorbula gibba are able to survive long periods at near anoxic conditions. After 57 days, 9 out of 14 specimens survived 10 -11°C and oxygen levels of 0.18 to 0.37 mg oxygen per dm³ (Christensen, 1970).
Burrowing. Varicorbula gibba is a shallow burrowing bivalve with very short siphons (Yonge & Thompson, 1976). When placed on their normal substratum, individuals extrude their long, thin, foot to a distance that may exceed the length of its shell (Yonge, 1946). The process of burrowing is very slow. For example, an individual 1 cm long took about 30 minutes to burrow below the surface. This is slow when compared to other bivalve species, for example Abra alba that can disappear below the surface in less than a minute. It is the stout rounded shell that makes slow progress into the substratum, whereas Abra alba has a much-flattened shell and foot, therefore, slides quickly into the substrata (Yonge, 1946).
Predators. Varicorbula gibba is consumed by gastropods, crustaceans, fish and echinoderms. Predators of Varicorbula gibba include the necklace shell Natica poliana (Jones, 1956), the sand star Astropecten irregularis (Christensen, 1970), the brittle star Ophiura texturata, the common starfish Asterias rubens, the common shore crab Carcinus maenas and the brown shrimp Crangon crangon (Jensen, 1988).
Non-native species. In Australia, Varicorbula gibba is an alien species and a pest (CRIMP, 2000). Varicorbula gibba is now widespread and highly abundant in Port Phillip Bay (Australia) (Talman, 1998; cited in Talman & Keough, 2001). Varicorbula gibba might affect endemic Australian species via habitat modification, predation on planktonic larvae, and competition. It also possesses a number of characteristics that may give it a competitive advantage over Australian endemic species, such as the capacity for fast growth and the ability to tolerate a wide range of environmental conditions including anoxia and eutrophication (Jensen, 1990; Talman & Keough, 2001). Concern has arisen in Australia regarding the impact of Varicorbula gibba on the commercial scallop Pecten fumatus. Varicorbula gibba and Pecten fumatus overlap in distribution, and as suspension feeders, it has been suggested that they utilize similar food and therefore may be competing for space and food (Talman & Keough, 2001). It was found that ambient densities of Varicorbula gibba had a significant impact on the size and growth of the native juvenile Pecten fumatus but not on mortality rates (Talman & Keough, 2001). Scallops in the presence of Varicorbula gibba had shells that were, on average, 35% lighter, 24% smaller and exhibited 54% less growth (based on caging experiments) (Talman, 2000: cited in NIMPIS, 2002). As a result of these concerns, Australian authorities have developed new methods to control the spread of Varicorbula gibba. However, measures such as dredging, beam trawling, mopping, changing salinity and oxygen deprivation have all proved relatively unsuccessful (McEnnulty et al., 2001a).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Estuary, Offshore seabed, Open coast, Strait or Sound |
| Biological zone preferences | Lower circalittoral, Lower eulittoral, Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Gravel / shingle, Mixed, Muddy gravel, Muddy sand, Sandy mud |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed, Sheltered, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu), Reduced (18-30 psu), See additional Information, Variable (18-40 psu) |
| Depth range | ELW - 146 m |
| Other preferences | |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Global distribution. Varicorbula gibba has spread outside its native range. This species and its larvae can survive long periods in ballast water and can generate heavy or at least significant populations in foreign harbours. It is most likely that the presence of larvae in ballast water has resulted in the introduction of Varicorbula gibba into Australian waters in Port Phillip Bay (McEnnulty et al., 2001b). Its occurrence in Port Phillip was the first documented record of the species outside its area of natural distribution (Talman & Keough, 2001). The clam has subsequently been found in Portland, Western Port Bay in Victoria, Devonport and the D'Entrecasteaux Channel in Tasmania (CRIMP, 2000).
Substrata. Varicorbula gibba is specialized for life in a substratum of muddy sand mixed with larger pieces of gravel and stone that are necessary for the planting of its single byssus thread (Yonge, 1946). This preference for muddy substrata was reported by Jones (1956). Jones (1956) recorded significant differences in the numbers of Varicorbula gibba between two sites in Port Erin on the Isle of Man. Higher numbers of Varicorbula gibba were recorded in areas of coarse muddy sand. In an area only 1/2 mile seaward from the previous site the sediments were fine and the numbers of Varicorbula gibba present were low (Jones, 1956). In the Adriatic Varicorbula gibba was completely absent in clean, sandy bottoms as it prefers some mud (Hrs-Brenko, 1981). Preference for coarse muddy sand was also seen in Port Philip Bay where Varicorbula gibba are rarely found in sediments that contain less than 10% mud (<63 µm). Below 15% mud, there was a strong relationship between the percentage of mud and the abundance of Varicorbula gibba (Parry & Cohen, 2001). Above 15% mud, there was no significant relationship between the abundance of Varicorbula gibba and the percentage of mud in the finer sediments (Parry & Cohen, 2001).
Water quality. Hrs-Brenko (1981) suggested that Varicorbula gibba thrives in eutrophic waters.
Salinity range. Varicorbula gibba has been recorded at the following salinities, 26 to 39 ppt in Port Phillip Bay (Talman, 2000: cited in NIMPIS, 2002), 28 to 34 ppt in Limfjord, Denmark, (Jensen, 1990), 27 to 32 ppt in Nissum Bredning, Denmark (Jensen, 1988) and 8.2 to 38.6 ppt in Elefsis Bay, Greece (Theodorou, 1994).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | No information |
| Generation time | See additional information |
| Age at maturity | Insufficient information |
| Season | Summer - Autumn |
| Life span | 1-2 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | See additional information |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
Reproduction. Yonge (1946) determined that Varicorbula gibba was dioecious with no evidence of a sex change. When the gonads of Varicorbula gibba are maturing it is easy to distinguish between males and females. Males have white testes whereas females have pink ovaries (Yonge, 1946). Reproduction and spawning generally occur during summer and autumn. Yonge (1946), observed that during early August the male gonads were filling but were not yet ripe and that the testes had developed further than the ovaries. However, no active sperm were present. In late August, female Varicorbula gibba the ovaries were filling. No ripe sperm was found until the middle of September. By the end of September, the male and female specimens were ripe. The ripe females had relatively large yolky eggs and ripe males had very active sperm (Yonge, 1946). Fosshagen, (1965; cited in Muss, 1973) found larvae of Varicorbula gibba in plankton from October to November and once again from January to February and suggested that it was possible for large individuals to spawn during the autumn.
Larval settling time. The settling time of Varicorbula gibba larvae is variable depending on location and may take several months (Jensen, 1988). In Danish waters, settlement occurred in August. (Jensen, 1988) stated that the settlement of Varicorbula gibba was very distinct with very few specimens below 2 mm in size during the month of September in Limjford. The recruitment of Varicorbula gibba was achieved within one week after settlement (Jensen, 1988). However, high moralities of newly settled individuals occurred during the first month of settling. It was suggested that this may be due to predation from epibenthic predators. Low and constant mortality occurred during the winter months with decreases in abundance again in spring and early summer. It was suggested that these observations could be due to the weakened conditions in the bivalves that had spawned (Jensen, 1988).
Longevity. Jensen (1990) suggested that the lifespan of Varicorbula gibba seemed to be shorter, at one to two years in Nissum Bredning, than in the first part of this century when individuals had a lifespan of five to six years with a maximum size of 12 mm (Jensen 1919: cited in Jensen, 1990).
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceVaricorbula gibba lives infaunally in muddy sandy sediments. Removal of the substratum would also remove the entire population of this species, and so the intolerance has been assessed to be high with a moderate recoverability. See additional information for recoverability. | High | High | Moderate | Moderate |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceVaricorbula gibba is a burrower in shallow muddy or sandy sediments and uses a byssus thread to attach to pieces of shell or rock in the sediment (CRIMP, 2000). It uses its short inhalant siphon above the sediment for feeding and respiration. If smothered Varicorbula gibba would most likely burrow up through the new sediment. Varicorbula gibba is also considered to be generally tolerant of prolonged oxygen deprivation (see deoxygenation below). Laboratory studies on Varicorbula have shown that they can survive up to 57 days in near anoxic conditions (Jensen, 1990). Therefore Varicorbula could probably survive for 1 month under smothering conditions (see benchmark). However, sudden smothering of the sediment would halt feeding. Therefore, intolerance has been assessed as low with an immediate recoverability level. | Low | Immediate | Not sensitive | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceLevels of suspended sediment are likely to be most relevant to feeding. An increase in suspended sediment is likely to increase the rate of siltation and the availability of food. This will affect Varicorbula as it is a suspension feeder. However, Varicorbula is one of the most efficient bivalve particle feeders (Kiørboe & Mohlenberg, 1981). In the Limfjord, increased winds caused a greater mixing of the water column and probably greater resuspension of bottom material in 1986. As a result population increases were recorded with higher densities and faster growth than the previous year when wind speeds were not as high (Jensen, 1990). Varicorbula gibba also has a well developed cleansing mechanism to deal with the accumulation of pseudofaeces by posterior and periodic contractions of the quick muscle component in the adductors (Yonge & Thompson, 1976). An increase in sediment may benefit Varicorbula gibba and tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceLevels of suspended sediment are likely to be most relevant to feeding. A decrease in suspended sediment is likely to decrease the availability of food for suspension feeders like Varicorbula gibba. Mortality is unlikely to occur within a month (see benchmark) but growth rates may be slower and so intolerance is assessed as low. When suspended sediment levels return to normal so to will food availability and recoverability is assessed as immediate. | Low | Immediate | Not sensitive | Low |
Desiccation [Show more]Desiccation
EvidenceThe effect of desiccation stress on Varicorbula gibba is likely to be minimal as it lives infaunally in muddy sand and is able to burrow to avoid or reduce the effects of desiccation. Bivalves are also able to respond to desiccation stress by valve adduction during periods of emersion. Nevertheless, some stress is likely and an intolerance of low has been recorded but with a very high recoverability. | Low | Very high | Very Low | Low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAn increase in emergence may cause thermal stress on Varicorbula gibba and increase the risk of dislodgement from the sediments because of the increased strength of wave action, especially in shallow water populations. Therefore, an intolerance of intermediate is given with a high recoverability. | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA decrease in emergence is not likely to stress Varicorbula gibba and may benefit the species, allowing Varicorbula gibba to colonize further up the shore and increase its habitat range. Periods of thermal stress, risk of predation and dislodgement would be reduced. Therefore, tolerant* is recorded. | Tolerant* | Not relevant | Not sensitive* | Low |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAn increase in the water flow rate would increase the availability of food that may increase growth rates and the size of individual Varicorbula gibba. However, increased water flow may cause the substratum to be disturbed and the sediment on the seabed to erode. This scouring of sand and gravel causes coarse sediments to become unstable and difficult to burrow perhaps leading to the dislodgement and abrasion of Corbula gibba. The sediments and the species within may then be transported to another area. High water flow rates may also damage or prevent the settlement of larvae that can lower recruitment levels and lower the population present (Hiscock, 1983). Therefore, an intolerance of intermediate has been assessed with high recoverability. | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceA decrease in the water flow rate could result in a reduction in food that may be obtained from suspension feeding in Varicorbula gibba, which could lower growth rates and the sizes of individuals within the population. It may also lower the dispersion of planktonic larvae. In areas exposed to less water flow, the sediments will be more stable (Hiscock, 1983) and particles may become finer and the substratum may become more muddy which is the preferred substrata of Varicorbula gibba. However, a decrease in water flow over the benchmark level of 1 year may also cause the substratum to become too muddy for Varicorbula gibba, which prefers sediments that contain between 10 - 15 % mud (Parry & Cohen, 2001). Therefore, intolerance has been assessed as intermediate with a high recoverability level. | Intermediate | High | Low | Low |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceVaricorbula is present in Mediterranean and Australian waters. Growth has been recorded at the following temperatures:
Therefore, Varicorbula gibba has been assessed as tolerant to increases in temperature at the benchmark level. | Tolerant | Not relevant | Not sensitive | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceVaricorbula gibba has a wide geographic range, occurring in waters throughout the British Isles and is likely to be tolerant of lower temperatures than it experiences in Britain and Ireland. Varicorbula gibba would probably tolerate a decrease in temperature (see benchmark) as it has been found at temperatures between - 1 to 16 °C in the Limfjord (Jensen, 1990), and also at 7.5 °C in the Kattegat (Christensen, 1970). Therefore, Varicorbula gibba has been assessed to be tolerant to decreases in temperature. | Tolerant | Not relevant | Not sensitive | |
Increase in turbidity [Show more]Increase in turbidity
EvidenceVaricorbula gibba does not require light therefore, the effects of increased turbidity on light attenuation are not directly relevant. An increase in turbidity may, however, affect primary production in the water column that would lower phytoplankton availability for Varicorbula gibba, as it is a suspension feeder, but it can use other food sources e.g. particulate organic matter. Therefore, intolerance has been assessed as low with a very high recoverability. | Low | Very high | Very Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceVaricorbula gibba does not require light therefore, the effects of a decrease in turbidity on light attenuation are not directly relevant. A decrease in turbidity may however, affect primary production in the water column that would increase phytoplankton availability for Varicorbula gibba. That could improve the growth rates of Corbula gibba and also increase their abundance. A decrease in turbidity is unlikely to affect Varicorbula gibba. Therefore not relevant has been recorded. | Not relevant | Not relevant | Not relevant | Low |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceVaricorbula gibba inhabits coarse muddy / sandy environments. This preference for coarse muddy sands was observed offshore of Port Erin on the Isle of Man where significant differences in the numbers of Varicorbula gibba was recorded. In areas where the substratum was coarse, the numbers of Corbula gibba were abundant. Whereas in areas where the substratum was fine the abundance of Varicorbula gibba was low (Jones, 1956).
| Intermediate | High | Low | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceChanges in wave exposure are likely to have marked effects on the sediment dynamics. If the wave exposure is decreased sediments that are deposited will slowly consolidate becoming more fine and muddy and can increase the substratum. Decreased exposure could increase siltation and the risk of smothering. Varicorbula gibba is specialized for its preferred habitat of muddy sand, however, a decrease in wave exposure over the benchmark period of 1 year may cause the substrata to become too muddy for Varicorbula gibba. Therefore, intolerance has been assessed as intermediate with a high recoverability. | Intermediate | High | Low | Moderate |
Noise [Show more]Noise
EvidenceNo information was found concerning the intolerance levels of Varicorbula gibba to noise. This species is not expected to be sensitive to the level of the benchmark. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceVaricorbula gibba probably has little visual acuity and was recorded to be not sensitive to this factor. | Tolerant | No information | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceVaricorbula gibba has a small solid shell. The shells of Varicorbula gibba may be vulnerable to physical damage (from e.g. otter boards) (Rumohr & Krost, 1991). However, the size of Varicorbula gibba relative to the meshes of commercial trawls may ensure survival of a moderate proportion of disturbed individuals that pass through them. Specimens exposed on the sediment surface would be at risk of predation. Bergmann & van Santbrink (2000) reported direct mortalities of <0.5%, 9% and 14% from the passage of an experimental beam trawl, depending on the type of trawl used and sampling method employed. They noted that smaller species or smaller individuals of larger species suffered lower mortalities. Overall, they concluded that Varicorbula gibba was amongst the species studied that were relatively resistant to bottom trawling (Bergmann & van Santbrink, 2000). | Intermediate | High | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceFishing for demersal species will disturb the surface layer of sediment and any protruding or shallow burrowing species. The small size of Corbula gibba may ensure that individuals are sieved over the mesh of fishing nets. Once through the net Varicorbula gibba are then able to resettle in the substrata. Displacement may also occur during storms if the sediment is mobilized. The increased wave action may cause whole populations to be lifted along with the substratum and transported by sediment bedload transport to a different area. Varicorbula gibba can burrow back down into the sediment when it is displaced to the surface therefore, it is probably relatively tolerant of displacement. However, it burrows slowly and when displaced the risk of predation by predators is increased which can lead to some mortalities of Varicorbula gibba. Therefore, an intolerance of intermediate has been recorded with a high recoverability. | Intermediate | High | Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceNo specific information was found concerning the effect of synthetic chemicals on Varicorbula gibba. However, an inference may be drawn from related species. Burrowing and avoidance behaviour in the bivalves Tellina tenuis and Macoma balthica becomes impaired when they are exposed to phenol but no deaths occurred. Impairment of burrowing can leave bivalves vulnerable to predation and wave action (Møhlen & Kiørboe, 1983). High levels of tributyltin (TBT), the toxic component of many antifouling paints, has been implicated in the slow growth and shell malformation 'balling' in the oyster Magallana gigas and larval mortality in Mytilus edulis (Beaumont et al., 1989). Overall, an intolerance of intermediate has been suggested, albeit with very low confidence. | Intermediate | High | Low | Very low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceHeavy metals can inhibit the activity of many enzymes and affect the function of several cellular constituents such as membranes, they can also inhibit growth, the production of the byssal thread, respiration, filtration rate, protein synthesis, the uptake of amino acids by various tissues and compromise reproduction in bivalves (full review by Aberkali & Trueman, 1985). The embryonic and larval stages of bivalves are the most vulnerable to heavy metals (Bryan, 1984).
Bryan (1984), states that Hg is the most toxic metal to bivalve molluscs in experimental studies while copper (Cu), cadmium (Cd) and zinc (Zn) seem to be most problematic for bivalves in the field. For example:
Rygg (1985) used 71 sampling stations in a dozen fjord areas with varying degrees of pollution to examine the effects of pollution on benthic fauna. He noted that benthic faunal biodiversity decreased with increasing Cu concentrations in the sediment. Corbula gibba was reported to be present at some but not all of the stations where sediment copper concentrations were above 200 ppm in the sediments and was classified as one of the moderately tolerant species (Rygg, 1985). A concentration of 200 ppm was approximately 10 tens background values (Rygg, 1985). Corbula gibba was more tolerant than the bivalves, Ennucula tenuis, which was absent at all the sampling stations and Thyasira equalis, which was occasionally present at the sampling stations (Rygg, 1985). Overall, an intolerance of intermediate has been recorded, since Corbula gibba was exclude from some of the polluted sites examined.. | Intermediate | High | Low | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceHydrocarbons may produce substantially reduced feeding, respiration and energy metabolism rates that reduce growth and reproduction as observed in Mya arenaria (Cooper & Cristini, 1994) and Mytilus edulis (Moore et al., 1987). Reproduction may also be compromised on exposure to hydrocarbons, for example Macoma balthica showed gamete resorption and abnormal gamete development. Additional effects of hydrocarbons on bivalves include a decline in tissue and shell growth, increased susceptibility to predation, parasitism and disease (Moore et al., 1987).
However, oil spills may benefit some bivalve molluscs. For instance, the 1978 Amoco Cadiz oil spill may have benefited the population of Abra alba present due to the nutrient enrichment that was caused by the oil spill. The biomass of the community doubled as a result of an increase in Abra alba abundance following the oil spill. Throughout the 20 years of monitoring the community's recovery, Abra alba has been one of the dominant species recorded (Dauvin, 1998). Corbula gibba has been noted as being indifferent to organic pollution (Pearson & Rosenberg, 1978) and has been recorded to often thrive in nutrient enriched waters (Crema et al., 1991) (see nutrients below). However, no information on the effects of hydrocarbon contamination on Corbula gibba was found, and intolerance has not been assessed. | No information | No information | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo specific information was found concerning the effects of radionuclides on Varicorbula gibba. | No information | No information | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceVaricorbula gibba is known to be a pioneer species in recolonization of defaunated seabeds and prominent in subnormal zones in areas polluted or enriched by organic material (Pearson & Rosenberg, 1978; Jensen 1990). It has also been suggested that Corbula gibba are indicative of unstable environments such as ones with low oxygen levels and areas of eutrophication (Crema et al., 1991). For example, samples were taken of the macrozoobenthic community in Elefsis Bay in the northern Adriatic in 1985. Elefsis Bay suffered from nutrient enrichment where nutrient pollution mainly came from the disposal of untreated waste waters of about 600,000 m³ / day at the entrance of the bay. The major nutrient inputs recorded were from phosphates, silicates, nitrites, nitrates and ammonium. Eutrophication of the northern Adriatic Sea was marked by red tides, extensive mucus aggregates, anoxic bottom conditions and mass mortalities. Despite this anoxia, large abundance's of Varicorbula gibba were recorded. The abundance of Varicorbula gibba at one sampling station was 1396 ind/m² and during the summer of 1989 Varicorbula gibba was the only living species recorded (Theodorou, 1994). Nutrient enrichments appear to benefit Corbula gibba by allowing it to increase its population size and to further colonize an area. It was suggested that as the amount of organic material reaching sediments increases, the larger species and deeper burrowing species are gradually eliminated and replaced by greater numbers of bivalves like Varicorbula gibba (Pearson & Rosenberg, 1978). Therefore, nutrient enrichment may benefit Varicorbula gibba and tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Increase in salinity [Show more]Increase in salinity
EvidenceVaricorbula gibba are mainly found at oceanic salinities but have also been recorded in 26 - 39 ppt in Port Phillip Bay (Talman, 2000: cited in NIMPIS, 2002). Therefore, it is likely that Varicorbula gibba would tolerate an increase in salinity at the benchmark level. | Tolerant | Not relevant | Not sensitive | Very low |
Decrease in salinity [Show more]Decrease in salinity
EvidenceVaricorbula gibba are found at oceanic salinities and in estuarine waters showing a tolerance for a reduction in salinity. In Elefsis Bay, Corbula gibba can be found at salinities as low as 8.2 ppt (Theodorou, 1994). Therefore Corbula gibba is likely to be tolerant of decreases in salinity. | Tolerant | Not relevant | Not sensitive | |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceDiaz & Rosenberg (1995) state that Varicorbula gibba is resistant to severe hypoxia. Varicorbula gibba is often found at the edge of anoxic and azoic areas (Pearson & Rosenberg, 1978) and it has been suggested that it is highly tolerant to environmental variability (Rosenberg, 1997).
Varicorbula gibba has shown tolerance to severe decreases in oxygenation therefore, an intolerance assessment of low has been given with a recoverability assessment of immediate. | Low | Immediate | Not sensitive | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceThe ciliate Sphenophrya dosiniae has been found living in specimens of Varicorbula gibba. If a lamellibranch is infected with Sphenophrya dosiniae, the ciliates will always occur in great numbers in the mantle cavity of their host (Fenchel, 1965). Sphenophrya dosiniae was found in 40 % of the specimens of Varicorbula gibba in the Gullmarfjord (Fenchel, 1965). No specific information concerning the effects of these ciliates on Varicorbula gibba was found. However, in the bivalve Crassostrea virginica, Sphenophrya dosiniae induced the formation of a lump known as a 'xenoma' that contains hundreds of ciliates (Weissenberg, 1922; cited in Laucker, 1983). Neither the ciliates or the xenomas appeared to distress Crassostrea virginica. However, parasitic infections are likely to result in sub-lethal effects and an intolerance of low has been recorded with a very high recoverability. | Low | Very high | Very Low | Low |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceThere is no evidence of adverse effects or competition from non-native species on Varicorbula gibba. Therefore, an intolerance and recoverability assessment could not be made. | No information | No information | No information | Low |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceVaricorbula gibba are not targeted for extraction. Therefore, an intolerance assessment is not relevant. | Not relevant | Not relevant | Not relevant | Low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceBergmann & van Santbrink (2000) reported direct mortalities of <0.5%, 9% and 14%from the passage of an experimental beam trawl, depending on the type of trawl used and sampling method employed. They noted that smaller species or smaller individuals of larger species suffered lower mortalities. Overall, they concluded that Varicorbula gibba was amongst the species studied that were relatively resistant to bottom trawling (Bergmann & van Santbrink, 2000). | Intermediate | High | Low | Moderate |
Additional information
Recoverability. The life span for individuals of Varicorbula gibba is about one to two years (CRIMP, 2000). It has a rapid growth rate in the first few months of its life and the ability to survive in a wide range of environmental conditions and the capacity to achieve high population densities (Jensen, 1990). The settling time of Varicorbula gibba larvae is variable and may change depending on location and may take several months (Jensen, 1988). In Danish waters, there were high moralities of newly settled individuals during the first month of settling. It was suggested that this was maybe due to predation from epibenthic predators (Jensen, 1988). Jensen (1988) reported that the survival rate of juveniles was around 19 to 31% in Limjford. This was followed by low and constant mortality during the winter months and decreases in abundance again in spring and early summer. Jensen (1988) suggested that it could be due to the weakened conditions in the bivalves that had spawned. Despite the juvenile mortalities, high densities of adult Varicorbula gibba still occurred as Varicorbula gibba can produce a large number of eggs. Jensen (1988), stated that in Danish waters the recruitment of Varicorbula gibba was achieved within one week after settlement.
Recruitment may be sporadic, and prolonged long-term variations in the abundance of Varicorbula gibba may occur. For example, a high abundance of Corbula gibba was recorded (about 1,500 /m²) between 1910 and 1935. This was followed by a constant low abundance (about 100 /m²) until 1952 when abundances rose again (Jensen, 1988).
Varicorbula gibba is known to be a pioneer species in the recolonization of defaunated seabeds and the species is abundant in subnormal zones in areas polluted or enriched by organic material (Pearson & Rosenberg, 1978; Jensen, 1990). Overall it is likely that this species has good powers of population recovery. A population that is reduced in extent or abundance could potentially recover within a few years, depending on recruitment. Its ability to recolonize defaunated areas suggests that the population would recover in a relatively short period of time even if the population was removed.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Supports which species. Rasmussen (1973), suggests that Varicorbula gibba are important as food for eels and flatfishes in Danish waters and may form part of the food of young fish.
Introduction of Varicorbula gibba to Australian waters. Varicorbula gibba is an alien species and a pest (CRIMP, 2000) in Australian waters. Varicorbula gibba is now widespread and highly abundant in Port Phillip Bay, (Australia) (Talman, 1998; cited in Talman & Keough, 2001). Varicorbula gibba possesses a number of characteristics that may give it a competitive advantage over Australian endemic species, such as the capacity for fast growth and the ability to tolerate a wide range of environmental conditions including anoxia, and eutrophication (Jensen, 1990; Talman & Keough, 2001). Concern has arisen in Australia for one native species the commercial scallop Pecten fumatus. Varicorbula gibba and Pecten fumatus overlap in distribution, and as suspension feeders, they presumably utilize similar food.
Bibliography
Aberkali, H.B. & Trueman, E.R., 1985. Effects of environmental stress on marine bivalve molluscs. Advances in Marine Biology, 22, 101-198.
Ball, B., Munday, B. & Tuck, I., 2000b. Effects of otter trawling on the benthos and environment in muddy sediments. In: Effects of fishing on non-target species and habitats, (eds. Kaiser, M.J. & de Groot, S.J.), pp 69-82. Oxford: Blackwell Science.
Beaumont, A.R., Newman, P.B., Mills, D.K., Waldock, M.J., Miller, D. & Waite, M.E., 1989. Sandy-substrate microcosm studies on tributyl tin (TBT) toxicity to marine organisms. Scientia Marina, 53, 737-743.
Bergman, M.J.N. & Van Santbrink, J.W., 2000b. Fishing mortality of populations of megafauna in sandy sediments. In The effects of fishing on non-target species and habitats (ed. M.J. Kaiser & S.J de Groot), 49-68. Oxford: Blackwell Science.
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Christensen, A.M., 1970. Feeding biology of the sea star Astropecten irregularis. Ophelia, 8 (1), 1-134. DOI https://doi.org/10.1080/00785326.1970.10429554
Cooper, K. & Cristini, A., 1994. The effects of oil spills on bivalve molluscs and blue crabs. In Before and After an Oil Spill (ed. J. Burger), pp. 142 -159. New Brunswick, New Jersey: Rutgers University Press.
Crema, R., Castelli, A. & Prevedelli, D., 1991. Long term eutrophication effects on macrofaunal communities in Northern Adriatic Sea. Marine Pollution Bulletin, 22, 503 - 508.
CRIMP (Centre for Research on Introduced Marine Pests)., 2000. Marine Pest Information Sheet, European Clam (Corbula gibba), Infosheet No. 8. [Online]. [cited on 2004-01-21].
Crompton, T.R., 1997. Toxicants in the aqueous ecosystem. New York: John Wiley & Sons.
Dauvin, J.C., 1998. The fine sand Abra alba community of the Bay of Morlaix twenty years after the Amoco Cadiz oil spill. Marine Pollution Bulletin, 36, 669-676.
Diaz, R.J. & Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
Fenchel, T., 1965. Ciliates from Scandinavian Molluscs. Ophelia, 2, 71 - 174.
Hartnoll, R.G., 1983. Substratum. In Sublittoral ecology. The ecology of the shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 97-124. Oxford: Clarendon Press.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Healy, J.M. & Lamprell, K.L., 1996. The Atlantic - Mediterranean bivalve, Corbula gibba (Olivi) (Corbulidae: Myoidea) in Port Phillip Bay, Victoria. Memoirs of the Queensland Museum, 39, 315-331.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Hrs-Brenko, M., 1981. Population studies of Corbula gibba (Olivi), Bivalvia, Corbulidae, in the Northern Adriatic Sea. Journal of Molluscan Studies, 47, 17 - 24.
Jensen, J., 1990. Increased abundance and growth of the suspension feeding bivalve Corbula gibba in a shallow part of the eutrophic Limfjord Denmark. Netherlands Journal of Sea Research, 27, 101-108.
Jensen, J.N., 1988. Recruitment, growth and mortality of juvenile Corbula gibba and Abra alba in the Limfjord, Denmark. The Baltic Sea environment: history, eutrophication, recruitment and toxicology. Kieler Meeresforschungen (Sonderheft), 6, 357-365.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Jones, N. S., 1956. The fauna and biomass of a muddy sand deposit off Port Erin, Isle of Man. Journal of Animal Ecology, 25, 217 - 252.
Karlson, K., Rosenberg, R. & Bonsdorff, E., 2002. Temporal and spatial large - scale effects of eutrophication and oxygen deficiency on benthic fauna in scandinavian and baltic waters - a review. Oceanography and Marine Biology: an Annual Review, 40, 427-489.
Kitching, J. A., Ebling, F. J., Gamble, J. C., Hoare, R., McLeod, A. A. Q. R. and Norton T. A., 1976. The ecology of Lough Ine XIX. Seasonal changes in the western trough. Journal of Animal Ecology, 45, 731 - 758.
Kiørboe, T. & Møhlenberg, F., 1981. Particle selection in suspension-feeding bivalves. Marine Ecology Progress Series, 5, 291-296.
Lauckner, G., 1983. Diseases of Mollusca: Bivalvia. In Diseases of marine animals. Vol. II. Introduction, Bivalvia to Scaphopoda (ed. O. Kinne), pp. 477-961. Hamburg: Biologische Anstalt Helgoland.
Møhlenberg, F. & Kiørboe, T., 1983. Burrowing and avoidance behaviour in marine organisms exposed to pesticide-contaminated sediment. Marine Pollution Bulletin, 14 (2), 57-60.
McEnnulty, F. R., Bax, N. J., Schaffelke, B. and Campbell, M. L., 2001b. A review of rapid response options for the control of ABWMAC listed introduced marine pest species and related taxa in Australian waters. Centre for Research on Introduced Marine Pests, Technical Report No. 23. [On-Line] http://crimp.marine.csiro.au/reports/CRIMPTechReport23.pdf, 2004-01-27
McEnnulty, F.R., Jones, T.E. and Bax, N.J. , 2001a. The Web-based Rapid Response Toolbox [On-line] http://crimp.marine.csiro.au, 2004-02-03
Moodley, L., Heip, C.H.R. and Middelburg J.J., 1998. Benthic activity in sediments of the northwestern Adriatic Sea: sediment oxygen consumption, macro - and meiofauna dynamics, Journal of Sea Research, 40, 263 -280.
Moore, M.N.,Livingstone D.R., Widdows J., Lowe, D.M. & Pipe, R.K., 1987. Molecular, cellular and physiological effects of oil derived hydrocarbons on molluscs and their use in impact assessment. Philosophical Transactions of the Royal Society of London, 316. 603 - 623.
Muus, K., 1973. Settling, growth and mortality of young bivalves in the Øresund. Ophelia, 12, 79-116.
NIMPIS, (ed) Hewitt C.L., Martin R.B., Sliwa C., McEnnulty, F.R., Murphy, N.E., Jones T. and Cooper, S., 2002. Corbula gibba species summary. National Introduced Marine Pest Information System http://crimp.marine.csiro.au/nimpis, 2004-02-02
Oliver, P.G., Holmes, A.M., Killeen, I.J. & Turner, J.A., 2016. Marine Bivalve Shells of the British Isles. Amgueddfa Cymru - National Museum Wales. Available from: http://naturalhistory.museumwales.ac.uk/britishbivalves [Cited: 3 July 2018].
Parry, G.D. & Cohen, B.F., 2001. Exotic species established in Western Port, including an assessment of the status of the exotic species Corbula gibba, Alexandrium spp, Gymnodinium spp and Undaria pinnatifida. Report No. 45 [On-line] http://www.dse.vic.gov.au, 2004-01-28
Pearson, T.H. & Rosenberg, R., 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: an Annual Review, 16, 229-311.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Rasmussen, E., 1973. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia, 11, 1-507.
Rosenberg, R. & Loo, L., 1988. Marine eutrophication induced oxygen deficiency: effects on soft bottom fauna, western Sweden. Ophelia, 29, 213-225.
Rosenberg, R., 1997. Benthic macrofaunal dynamics, production and dispersion in an oxygen deficient estuary of west Sweden. Journal of Experimental Marine Biology and Ecology, 26, 107 - 133.
Rumohr, H. & Krost, P., 1991. Experimental evidence of damage to benthos by bottom trawling with special reference to Arctica islandica. Meeresforschung, 33 (4), 340-345.
Rygg, B., 1985. Effect of sediment copper on benthic fauna. Marine Ecology Progress Series, 25, 83-89.
Talman, S.G. & Keough, M.J., 2001. Impact of an exotic clam, Corbula gibba, on the commercial scallop Pecten fumatus in Port Phillip Bay, south-east Australia: evidence of resource-restricted growth in a subtidal environment. Marine Ecology Progress Series, 221, 135 - 143.
Tebble, N., 1976. British Bivalve Seashells. A Handbook for Identification, 2nd ed. Edinburgh: British Museum (Natural History), Her Majesty's Stationary Office.
Theodoeou, A.J., 1994. The ecological state of the Elefsis Bay prior to the operation of the Athens Sea outfall. Water, Science and Technology, 30, 161 - 171.
Thorson, G., 1946. Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddelelser fra Kommissionen for Danmarks Fiskeri- Og Havundersögelser, Serie: Plankton, 4, 1-523.
Yonge, C.M., 1946. On habits and adaptation of Aloidis (Corbula) gibba. Journal of the Marine Biological Association of the United Kingdom, 26, 358-376.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-02
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 17/04/2008