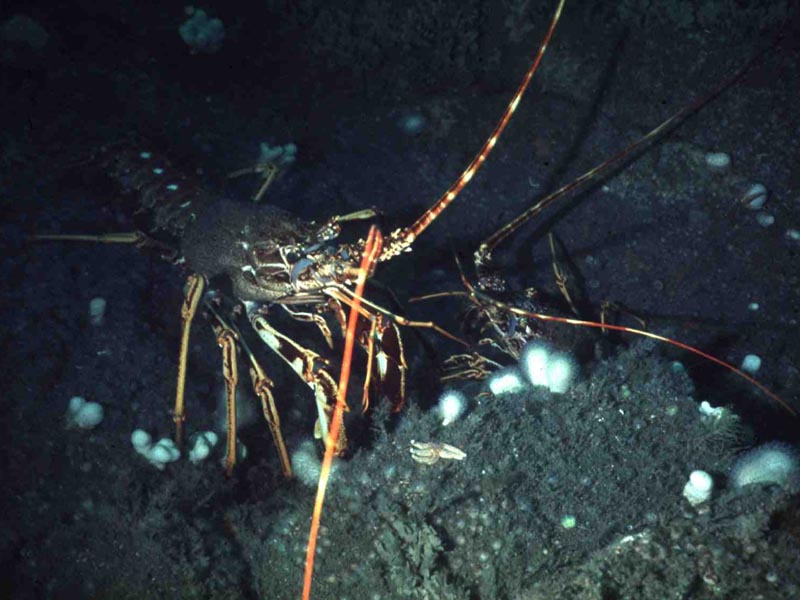
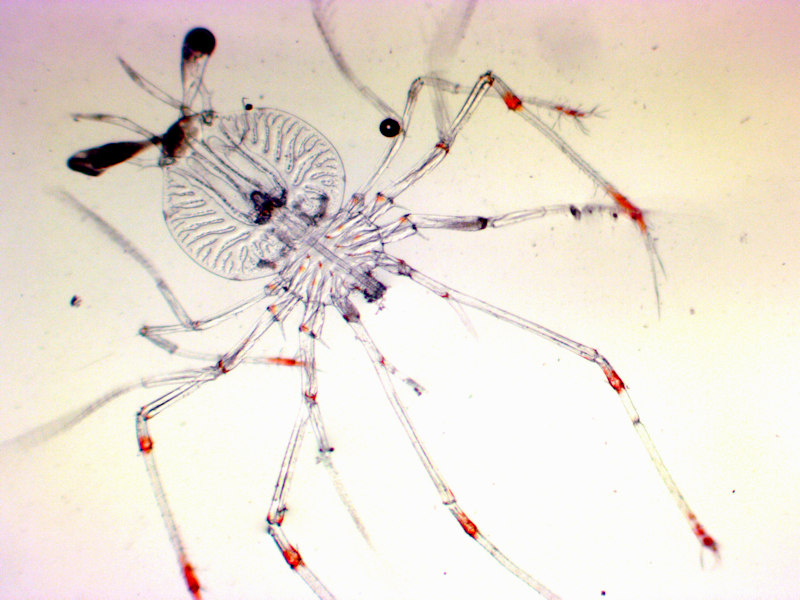
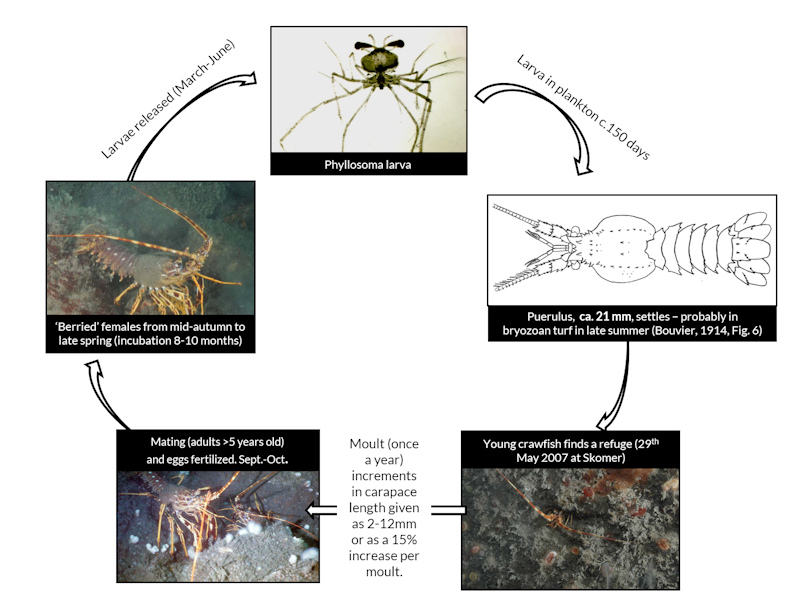
European spiny lobster (Palinurus elephas)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Eliza Gibson-Hall, Angus Jackson, Catherine Wilding & Charlotte Marshall | Refereed by | Dr Keith Hiscock |
| Authority | (Fabricius, 1787) | ||
| Other common names | Rock lobster, Crayfish, Crawfish | Synonyms | Palinurus vulgaris Gruvel, 1911 |
Summary
Description
A large spiny lobster, growing up to 60 cm in total length, with a stout, heavily armoured body. The colour is usually orange dorsally with darker spines and white underneath but brown, sandy and purple morphs are occasionally found (Hunter et al., 1996; Hunter, 1999). It has numerous sharp spines on the carapace, over much of the abdomen and on the larger appendages. There are two long antennae and small hook-like claws.
Recorded distribution in Britain and Ireland
The main populations are confined to the west coast of Scotland, the extreme south-west coasts of England & Wales and the west coast of Ireland. See 'additional information' in the Habitat section.
Global distribution
South and west coasts of the British Isles, south to the Azores, the western Mediterranean, Adriatic Sea and the Aegean Sea.
Habitat
Lives subtidally on rocky, exposed coasts in the circalittoral zone.
Depth range
5-400mIdentifying features
- Carapace covered with forward-directed spines; supra-orbital spines particularly prominent.
- Antennal stalks very heavy and spiny; flagellum stout, tapering and longer than the body.
- Typically orange colouration but may also be brown, sandy or purple.
- There are two large symmetrical white blotches on the tergites of somites 1-5, a single central blotch on the last segment and two blotches on the telson.
- Small hook-like claws.
Additional information
Also known in Britain as the crawfish, crayfish, spiny lobster or rock lobster, and the langouste rouge (red spiny lobster), langouste commune or langouste royale (royal spiny lobster) in France. Spiny lobsters were intensively fished by netting and diving in the late 1960s and early 1970s leading to local extinctions in south-west Britain in particular. First signs of recovery did not occur until 2014 when large numbers of recently settled individuals were seen in locations from where they had been absent for over 40 years. A similar recruitment was observed at the same time in Brittany. The newly settled lobsters have grown and recruitment has continued (Keith Hiscock, pers. comm.).
When placed in a tank with predator species Octopus vulgaris, Palinurus elephas undertook chemosensory behaviour to respond to the octopus odour (Gristina et al., 2011). They were subsequently able to detect the odour amongst a mixture of different odours and avoid the predator. Female lobsters ‘stridulate’. This is a creaking noise from the base of the antennae. as a result, they are known as ‘creakers’ in west Wales. The noise attracts males. The acoustic signals emitted by Palinurus elephas is within the range of ultrasonic frequencies (20-55 kHz) (Edmonds et al., 2016). Palero et al. (2008) found that the two northernmost Atlantic localities in their study (Western Scotland and Western Ireland) were clearly genetically differentiated from three other Atlantic localities (southwestern England, Brittany and the Bay of Biscay).
Listed by
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Arthropoda | Arthropods, joint-legged animals, e.g. insects, crustaceans & spiders |
| Class | Malacostraca | Crabs, lobsters, sand hoppers and sea slaters |
| Order | Decapoda | Crabs, shrimps, prawns, crayfish and lobsters |
| Family | Palinuridae | |
| Genus | Palinurus | |
| Authority | (Fabricius, 1787) | |
| Recent Synonyms | Palinurus vulgaris Gruvel, 1911 | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Data deficient | ||
| Male size range | 40-60 cm (TL) | ||
| Male size at maturity | 8-8.5 cm (CL) | ||
| Female size range | Medium-large(21-50 cm) (TL) | ||
| Female size at maturity | 7-8 cm (CL) | ||
| Growth form | Articulate | ||
| Growth rate | See additional information | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Crawler or Walker, Mobile | ||
| Characteristic feeding method | Scavenger, Searcher / forager | ||
| Diet/food source | Omnivore | ||
| Typically feeds on | Echinoderms, small gastropods and bivalves, microalgae, shrimp larvae, bryozoans, annelids. | ||
| Sociability | Gregarious | ||
| Environmental position | Epibenthic, Epifaunal | ||
| Dependency | Independent. | ||
| Supports | Substratum Encrusting species such as Circeis armonicana and Spirorbis spirorbis (tube worms), Anomia ephippium (saddle oyster) and Electra pilosa (a bryozoan). Stalked barnacles have been recorded from the antennae. | ||
| Is the species harmful? | No | ||
Biology information
Size. Size is measured as total length (TL) while carapace length (CL) is the measurement usually used for fisheries management. The maximum overall total length is about 60 cm, although more commonly 40 – 50 cm, and the carapace length of males and females ranges between 8.5-19.3 cm and 7.9-18 cm respectively. On average, larger maximum sizes have been recorded in the Atlantic populations compared to the Mediterranean. Sexual maturity in females has been shown to inhibit further growth. This is thought to be due to the reproductive investment (Follesa et al., 2007).
Growth. Growth rate and age of individuals still remains poorly known with some tagging studies of largish individuals suggesting growth rates of less than 1 mm CL/year, whilst others suggest 12 mm a moult. There are discrepancies in growth rate between the Mediterranean and Atlantic populations as well as between authors. Mercer (PhD thesis, 1973) found a mean annual size increase in females at 1.2 cm CL/year in an Irish population. This is marginally lower than males from the same population (1.22 cm CL/year) (Mercer, 1973; cited in Hunter, 1999). Other growth rate estimates suggest an increase of 2-14% of CL with each moult (Campillo & Amadei, 1978) or as low as 0.9 mm CL/year (Hiscock et al., 2011). Growth increments are also thought to decrease as individuals approach their maximum size and are likely to vary with temperature. Hunter (1999) suggested that growth rates were higher in warmer waters. However, this is contrary to a review by Goñi & Latrouite (2005) that found faster growth rates in the Atlantic than the Mediterranean. In Pembrokeshire, the largest adult recorded in recent surveys had a carapace length of 15 cm (Jones, 2012). If the growth rate is lower than predicted, this may have further implications on the exact age of individuals.
Moulting. In Britain and Ireland, females moult in late summer between July and September (Hepper, 1977; Ansell & Robb, 1977; Hunter et al., 1996). The moult cycle of males in Britain and Ireland seems to be less clear. Hunter et al. (1996) reported that males have a moult peak in September coinciding with the female moult. According to Hepper (1977) and Hunter (1999), male Palinurus elephas in Britain and Ireland moult mainly in the winter months, although Hunter (1999) also states that males moult throughout the year. Soft males have been found throughout the year with a higher abundance in winter (Gachardo, 2006). Juveniles moult around 8-12 times during their first year post-settlement. Each moult results in a 2 mm increase in CL (Groeneveld et al., 2013). Moulting frequency decreases with age and after sexual maturity is reached, especially in females (Hunter, 1991).
Mobility and sociability. Palinurus elephas is more active at night, particularly for foraging (Diaz et al., 2001; Goñi & Latrouite, 2005). These nocturnal movements are thought to be linked to the moon phase as catches of the spiny lobster are reduced under a full moon (Goñi et al., 2001). Very few individuals have been observed out of their shelter during daylight hours suggesting a strong circadian rhythm (Diaz et al., 2001). They leave their shelters for both feeding and reproduction (Goñi & Latrouite, 2005). Palinurus elephas typically crawls on the substratum but may swim occasionally. Adults are solitary or occur in pairs and small groups. Mercer (1973, cited in Hunter, 1999) describes the species as 'typically gregarious' (sociable). The homing ability of Palinurus elephas appears to be minimal with a range of only 7 m2. The majority (90%) of individuals return to their resident shelter after foraging at night and nocturnal activity was reported to start an hour before sunset and end an hour before sunrise (Groeneveld et al., 2013).
Migration. Migrations may be triggered by seasonal changes in sea temperature as well as light intensity (Miller, 1990). However, mass migrations appear to be missing in this species (Follesa et al., 2007). Instead, several observations suggest that the species makes active annual migrations to and from deeper water (Ansell & Robb, 1977; Hunter, 1999). In the Atlantic, females move to deeper waters during egg development and return inshore prior to egg hatching (Goñi et al., 2001). Males are also thought to make onshore-offshore migrations although the timing in relation to female migrations is contentious (Goñi et al., 2001). The populations off the west of Ireland (except perhaps for large males) move offshore into deeper waters late in the year to overwinter and return back inshore in spring (Mercer, 1973, cited in Hunter, 1999). Longer distances are travelled by sexually immature males and females (CL 5.4-8 cm) suggesting that sexually mature individuals are more resident/sedentary (Follesa et al., 2009). This is shown in the high site fidelity and limited movement of these species (Follesa et al., 2011) with movement mainly restricted to 2.5 km/yr (Goñi & Latrouite, 2005). However, individuals can forage beyond the boundaries of certain Marine Protected Areas making the species vulnerable to fishing in those areas.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Offshore seabed, Open coast |
| Biological zone preferences | Circalittoral, Data deficient |
| Substratum / habitat preferences | Artificial (man-made), Bedrock, Large to very large boulders, Small boulders, Wrecks |
| Tidal strength preferences | No information |
| Wave exposure preferences | Exposed, Extremely exposed, Very exposed, Very sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | 5-400m |
| Other preferences | Small individuals (CP < approx. 8 cm) shelter in fissures and holes. Larger individuals are seen under ledges, i.e. more open situations. |
| Migration Pattern | Seasonal (environment), Seasonal (reproduction) |
Habitat Information
Around Britain and Ireland, the species is recorded on western seaboards eastwards to the Isle of Wight including on the Welsh coast of the Irish Sea and all around Orkney and Shetland and south in the North Sea to the Scottish border. The occasional isolated record from the east coast of Scotland suggests that larvae penetrate into and survive in the North Sea, presumably during periods when North Atlantic oceanic water intrudes further round into the North Sea (Hepper, 1977).
The species is usually found from the open coast to offshore rock where salinity is likely to be full (30-40 psu) (Ceccaldi & Latrouite, 2000). The typical depth range of the species is between 5 and 70 m (Ansell & Robb, 1977; Ingle, 1997) although it has been recorded at depths of 160 m (Noel, 1999).
In Pembrokeshire, Palinurus elephas was observed at depths of between 11.9 m and 22.8 m. The majority of individuals (77%), were recorded at 14-18 m (below chart datum) (Jones, 2012). The largest density of Palinurus elephas was found in the habitats with the highest number of rocky reefs and crevices (Jones, 2012). No records were found at less rocky sites and the crawfish were usually found close together. The highest number recorded in one day at a single site was eight; all juveniles and five were found in a single gully together (Jones, 2012). All Palinurus elephas collected by Ansell and Robb (1977) were from areas of strong tidal currents on shallow inshore reefs that were interspersed with steep vertical rock faces. However, the species has also been recorded from finer sediments at the edge of Zostera marina beds in Salcombe Harbour (MBA, 1957). Juveniles have been found in seagrass beds (Giadone et al., 2006).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | See additional information |
| Generation time | Insufficient information |
| Age at maturity | Female: 4-5 years, Male: Variable |
| Season | July - October |
| Life span | 21-50 yrs |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | Phyllosoma, Puerulus |
| Larval/juvenile development | Oviparous |
| Duration of larval stage | 1-6 months |
| Larval dispersal potential | >1000m |
| Larval settlement period | Insufficient information |
Life history information
Lifespan. The maximum estimated lifespan is up to 25 years with reproductive maturity occurring at approximately 5 years in the Mediterranean (Marin 1987). In Pembrokeshire, the majority of individuals measured 10-12 cm and were estimated at 8-10 years old but juveniles were lacking (Jones, 2012).
Maturity. In a marine reserve in the Mediterranean, Goñi et al. (2003) found that female Palinurus elephas were able to reproduce at 7.6-7.7 cm CL and males were sexually mature at 8.25 cm CL. This size-specific fecundity was considered to be comparable to a lightly fished population off Ireland. In Britain and Ireland, size at maturity is generally larger (Hunter, 1999). In Cornwall, Hunter et al. (1996) reported the smallest berried female measured 9 cm CL, whereas in Wales the smallest berried female measured 12.1 cm CL. The mean size of female, male and berried female Palinurus elephas in Cornwall was reported as 12.5, 13.2 and 13.5 cm respectively, and 15.6, 13.9 and 13.9 cm respectively in Wales (Hunter et al., 1996). Mercer (1973, cited in Hunter, 1999) found that 50% of the female population reached maturity at 8.2-8.6 cm CL in Irish Palinurus elephas, the smallest measuring ca 7 cm CL.
Female Palinurus elephas are thought to usually be mature by the time they recruit into the fishery (Hunter et al., 1996). Females are defined as physiologically mature when their ovaries reach ‘stage 4’ or higher during the reproductive season (Marin, 1987). For females, the age at which maturity is reached is around 4-5 years (Goñi et al., 2003). The relationship between gonad width and body size is calculated to estimate maturity in males, however, it is difficult to estimate an average size and or age at which males reach maturity due to the degree of variation (Goñi et al., 2003).
Mating. Mating is usually preceded by a 'pre-mating' moult, which occurs up to four weeks earlier (Ceccaldi & Latrouite, 1994). When the female is ready to mate, she emits a specific noise (stridulation) that attracts a mate (Mercer, 1973, cited in Ceccaldi & Latrouite, 1994). This mating call can attract males from a distance of 15-20 m. The noise ceases when a suitably sized male reaches the female. The male then deposits a spermatophore below the genital opening of the female. Mating occurs between June and October in the Atlantic (Goñi & Latrouite, 2005), and females bearing spermatophores have been reported from August to October in Britain and Ireland (Hepper, 1977; Ansell & Robb, 1977; Hunter, et al., 1996; Hunter, 1999). In laboratory experiments, Ansell & Robb (1977) found that eggs were released 7-10 days after the deposition of the spermatophore. As the eggs are laid, the spermatophores are torn with the claw on the fifth pereiopods (Mercer, 1973, cited in Hunter et al., 1996), thereby fertilizing the eggs. Single males in breeding tanks usually mate with several females and spawning occurs on the day of mating (Kittaka, 1997). Eggs are retained on the abdomen of the female for eight months prior to hatching (Hunter, 1999; Mercer, 1973). Reducing the number of males in the population can result in the remaining males being unable to produce enough sperm to successfully fertilise. Sperm limitation can become an issue if large females are unable to find a suitable male to mate with either through reduced numbers of males due to fishing mortality or due to a lack of males of a suitable size with which to mate. Egg production can also be reduced due to an insufficient amount of sperm. Successful mating in crawfish only occurs when the mating pairs are similar in size (Mouat & Shelmerdine, 2012).
Fecundity. Fecundity in Palinurus elephas is influenced by the size of the female, with heavier specimens producing more eggs (Ceccaldi & Latrouite, 1994). Goñi et al. (2003) reported fecundity in a marine reserve in the western Mediterranean to be between ca 23,000 and 202,000 eggs. This is generally three to five times lower than fecundity in many other spiny lobster populations (Hunter, pers. comm. & Groeneveld et al., 2013). Palinurus elephas carry fewer eggs than other genera of the same family (Panulirus and Jasus) but the eggs of Palinurus elephas are larger. Females of 10.5-11 cm CL in size contribute the most to egg production of a population. Larger females produce, on average, five times more eggs and larger larvae compared to smaller females (Goñi et al., 2003). In contrast, reduced male size has not been shown to affect their mating success. Palinurus elephas is more vulnerable to stress during mating and egg production (Ansell & Robb, 1977). Mercer (1973) estimated a mean egg loss of 10% for an 8-month incubation period for Atlantic, mature, females. This suggests that the loss of eggs could be positively correlated with temperature due to a stress response.
Larval ecology. Incubation in the Atlantic is typically nine months, after which, the eggs hatch in early summer (Hunter, 1999). In Cornwall, eggs were laid in late summer/autumn and hatched the following spring / early summer (Hepper, 1977). Most eggs have hatched by June in Wales and Cornwall (Hunter et al., 1996). Hatching takes two to eight days (Hunter, 1999). In Scotland, hatching was thought to occur in April and May (Ansell & Robb, 1977). There is only one clutch per year. In the Mediterranean, incubation lasts for only five months, probably reflecting warmer water temperatures (Hunter, 1999).
Larvae measure 2.9-3.9 mm TL at hatching, and are larger than other Plainuridae species (Groeneveld et al., 2013). They hatch at a more advanced stage compared to Jasus and Panulirus species termed the Phyllosoma. Phyllosomas of Palinurus elephas are unique in their morphology and their life history. The 1st instar is around 2.8 mm long compared to 1.6-2 mm of Palinurus japonicus. The hatched Phyllosomas are similar in size to the 3rd instar of Palinurus japonicus. Heavy mortality (50-80%) occurred in the 1st instar larval stage in laboratory culture (Kittaka, 1997). In phyllosoma culture, long pereiopods were often injured. Lost pereiopods regrew at the following moult but regenerated ones were usually fragile and often lost, leading to a decrease in feeding activity. The exopods of the 3rd pereiopods are already elongated though still incomplete in the 1st instar of Palinurus.
Phyllosomas drift offshore during a long pelagic life and return inshore after many moults to metamorphose and settle in the puerulus stage. The phyllosoma larval stage was reported to be 5-6 months in the Mediterranean and 10-12 months in the Atlantic Ocean (Mercer, 1973). In culture, phyllosomas of Palinurus elephas metamorphosed into pueruli after 132-148 days, a short lifespan compared to 200-300 days in other species of spiny lobster. Lowering the water temperature of the cultures from 18-20°C to 15-17°C and feeding different types of bivalves did not bring any improvement to survival. Jellyfish were their preferred food (Kittaka, 1997). The advanced development at hatching and shorter phyllosoma development meant that Palinurus elephas was thought to be the preferred species for aquaculture. However, survival has been poor for the species in more than 10 years of the culture trials (Kittaka, 1997).
Settlement period. The pueruli (intermediate juvenile-larvae stage) settled between June and July in the western Mediterranean (Diaz et al., 2001). Temperature is a critical factor affecting settlement success (Diaz et al., 2001) with larvae settling in warmer months of both the Mediterranean and the Atlantic. Larvae prefer to settle at depths of between 10-15 m.
Population dynamics. Fishing techniques have changed from the use of pots and diving to trammel nets. These techniques are more efficient and caused a particular decline in population in the Atlantic Ocean (Goñi & Latrouite, 2005). Palinurus elephas species is generally targeted at depths between 50-150 m (Catanese et al., 2018). In order to buffer the impact on recruitment from overfishing, it is important to maintain populations with a broad range of age and size classes (Diaz et al., 2016). The species are also sensitive to displacement due to their lack of capacity to ‘home’ and relocate to their place of origin (Follesa et al., 2014).
Palinurus elephas is considered to be a bycatch of some 100 finfish fisheries. The genera Homarus, Cancer, Palinurus and Necora can all be caught using the same traps (Tully et al., 2003). The use of closed-season management appears to be the most effective currently (IUCN, 2018). Landings of Palinurus elephas have fallen since 1997 from 42 million tonnes (mt) in that year through 19 mt in 1998 to 15 mt in 1999, 2000, 2001 and 2002 (FAO, 2002). The Welsh pot-hauled crawfish fishery fell from 55,000 kg in 1979 to less than 500kg in 1995 (Hunter, 1999). According to the Dublin Marine Institute, overfishing via tangle nets during the 1970s is thought to have been the main cause of the stock collapse. The significance of this decline is highlighted in Davies(2016) who found, during his diving expeditions in the 1960s, that each time a crawfish was removed from a hole a new crawfish moved into the same hole. In St. Ives 64 crawfish were found in a single dive in the same decade and the best sighting by Davies’ three-man crew was 410 crawfish during a south Wales dive. Numbers in the Mediterranean have also been reduced (Campillo & Amadei, 1978). Reasons for the decline are also thought to be due to the use of more efficient capture mechanisms as seen in the Atlantic. Environmental conditions may also play an important role (Hiscock, pers. comm., based on observations in Russell, 1973 and Wilson, 1951).
Despite these declines, 215 individuals have been recorded around the south of England to Pembrokeshire and Jersey (SWME, 2016) since 2014. There was a range of sizes, from large to small including two juveniles. This suggested a potential for recruitment and recovery of the population (Jones, 2011). Higher numbers were also seen all around Cornwall, particularly Falmouth Bay (Slater, 2017). Whilst numbers fluctuated over the years, there was a low number of individuals until 2015 when there was a dramatic increase in crawfish sightings in southern England; up to 25 individuals in 2015. Many of these individuals were juvenile which means there is a potential for this growth to be sustained (Slater, 2015). Data from the Isles of Scilly (2014) tagging programme showed most recaptured individuals were male. Many of the recaptured males and females showed no growth after 100 days. This suggests a bias towards a male population around the Isles of Scilly. The low number of juveniles recaptured is also a cause for concern as it suggests low recruitment (IFCA, 2015).
Although the larvae have a potentially large dispersal range, genetic differentiation has been reported between the populations from Britany and UK as well as between the Atlantic and Mediterranean populations has been reported (Palero et al., 2011; Babbucci et al., 2010). This difference is due to a limited gene flow and suggests a restricted larval dispersal. The restriction is most likely caused by oceanographic factors such as the Gulf Stream and oceanic processes (e.g. currents) from the Atlantic Ocean. In addition, there is a limited exchange between the east and west Mediterranean populations. In the north-west Mediterranean basin, there is a mesoscale cyclonic gyre caused by the northern Balearic currents. This gyre may lead to larval retention within the particular area and, hence, population subdivision.
Sensitivity review
Resilience and recovery rates
Palinurus elephas is intolerant of removal by fisheries or any predation by other species (especially likely for juveniles) that might occur. Recovery at a location may be by migration, however, evidence has shown a lack of long distance travelled by the species once settled from the larval stage (see migration). Recovery after a loss will, therefore, most likely be from larval settlement, which (see ‘Population dynamics’ above) may be episodic with decadal gaps between significant recruitment events. This species grows relatively slowly compared to other crawfish and has a long life expectancy of around 25 years (Marin, 1978).
The species appears to have a strong circadian rhythm and therefore remains in their shelters for a majority of the day. This ability to seek out and remain in shelters for long periods of time allows the crawfish to protect itself from both fishing equipment and predators. Palinurus elephas are also able to survive up to a week with no food during their pre-moult stage. Therefore, they are able to remain in their shelters for long periods of time and avoid fishing or natural events such as storms. Palinurus elephas are opportunistic feeders. This means they have the ability to adapt their diet if a certain prey species becomes unavailable. Shelters also provide a refuge for newly settled larvae, allowing recruitment.
Palinurus elephas reproduces annually and the eggs are incubated by the female. Despite this, the fecundity of the species is three to five times lower than other species in the same genus (Hunter, pers. comm.). Palinurus elephas is most vulnerable to the impacts of stress during breeding. This means the species has to weigh up the potential detrimental cost against the potential for recruitment (Ansell & Robb, 1977). An increase in stress caused directly and indirectly by humans such as fishing and climate change may reduce survival rates of females during the breeding period.
In culture experiments, single males bred with multiple females (Kittaka, 1997). The selected male also needed to be of a similar size to the female to fertilise successfully. A reduction in both the number of males as well as the range in male sizes could cause a reduction in available, suited, males for mating. The reduction of males may also put pressure on the remaining males in the population to produce enough sperm, again limiting overall reproduction and therefore fecundity (Mouat & Shelmerdine, 2012).
Palinurus elephas females of 10.5-11 cm CL in size contribute the most to egg production of a population. Larger females produce, on average, five times more eggs and larger larvae compared to smaller females (Goñi et al. 2003). In contrast, reduced male size has not been shown to affect their mating success.
The larvae of Palinurus elephas hatches at a far more advanced and developed state compared to Jasus and Panulirus. Despite this, observations and attempts at culturing the phyllosomas are yet to be successful. In culture, the 1st instars suffered heavy mortality (50-80%), (Kittaka, 1997).
The long pelagic life of the larval stages increases the potential range for dispersal but also increases their vulnerability to predation and physical damage and, hence, potential mortality and reduced recruitment. Larvae also need to travel in a direction that takes them close to suitable habitats for settlement. Once near a suitable general location, larvae may use olfactory or auditory clues to find a final location to settle (see, for instance, Stanley et al., 2012). Evidence of population subdivisions between and within Atlantic and Mediterranean populations suggests that larval survival and recruitment are not as effective as their long pelagic life and advanced stage of development might suggest.
Following the major decline in numbers and reduced distributional occurrence in Palinurus elephas in parts of south-west Britain at least since the 1970s (see ‘Population dynamics’) the remarkable recovery that has occurred since about 2014 demonstrates the severity of the over-fishing that caused the decline and, most importantly, the long time before any potential for recovery. Small individuals were seen at Lundy and at Skomer in 2007, although no such individuals were observed in over 20 years previously (Hiscock et al., 2011). The recruitment that has occurred since 2014 (SWME, 2016) is large and suggests that Palinurus elephas has the ability to recover from local extinction or severe reduction in numbers. However, this recovery appears to be very slow and it has been approximately 40+ years since a recruitment of this scale has been reported (Hiscock et al., 2011).
Marine Protected Areas may also aid recovery. After eight years of reduced fishing pressure, the catch rates within the Columbretes Islands, Spain, reserve were much higher and had significantly increased after a six month closed season. Despite the lack of exact numbers, the population has shown the ability to recover with the reduction of fishing pressures (Goñi et al. 2001). Diaz et al. (2016) suggested that it is important to maintain populations with a broad range of age and size classes, in order to buffer the impact on recruitment from overfishing.
There has been a lack of recovery of Palinurus elephas for ca 30 years after the decline in populations due to overfishing. Hiscock et al. (2011) suggested that recruitment from the plankton was episodic and infrequent. The evidence of genetic subdivision (Palero et al., 2011; Babbucci et al., 2010) supports this view. The Recovery is also hindered by the low fecundity, slow growth rate, and limited section of the population (large females) reproducing (Mouat & Shelmerdine, 2012). In addition, adults are nocturnal and favour specific shelters so that mobility is limited and individuals from other populations are unlikely to repopulating areas from which the species is removed.
Resilience assessment. Recruitment to and recovery of populations is likely to be prolonged and/or episodic. Therefore, recovery from any loss of the population (i.e. a reduction in the extent or abundance, resistance is 'Medium' or 'Low') may take up to 25 years where the populations are sparsely distributed (e.g. in Britain). Hence, a resilience of 'Low' will be recorded. However, where the population is severely reduced in abundance or extent (i.e. resistance is 'None') a resilience of 'Very low' will be recorded.
Hydrological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Temperature increase (local) [Show more]Temperature increase (local)Benchmark. A 5°C increase in temperature for one month, or 2°C for one year. Further detail EvidencePalinurus elephas is found in temperate (North Sea) to warmer waters as far south as the western basin of the Mediterranean. Different populations may have different thermoregulatory and adaptive ability, due to a lack of overlap and gene flow between populations (Palero et al., 2011, Babbucci et al., 2010). Summer catches in Scotland were higher than winter catches (Ansell & Robb, 1977). This was due to the higher occurrence in shallower waters during periods of summer temperatures. An increase in temperature might encourage individuals to move into the warmer areas, based on summer migratory inshore activity, which would increase vulnerability to inshore catches. Larval settlement takes place within a very limited thermal window, centred around the warmest months in both the Mediterranean and Atlantic (Diaz et al., 2001). Settlement begins when the sea surface temperature increases mid- May and peaks in June-July. This is a relatively short settlement period. Increased temperatures could decrease the time taken to settle, reducing gene flow further. Mercer (1973) suggested that egg loss was positively correlated with an increase in temperature due to an apparent increase in stress on the berried female. Egg loss during incubation by the females was around 26-28 % in the Western Mediterranean, higher than the Atlantic Ocean (Goni et al., 2003). Acute temperature change in the Laboratory of the Marine Research and Aquaculture (LIMIA, Balearic Islands Government) saw temperatures rise to above 24°C which resulted in the mass mortality of Palinurus elephas (Mallol et al., 2014) suggesting a low resistance to a sudden increase in temperatures. Sea surface temperature around the UK is projected to increase between 1.5-4°C by 2098 (Goodwin et al., 2012). A study site in Ireland exposed to an increase of 0.3-0.5°C between 1950 and 2009 showed a substantial decline in abundance of Palinurus elephas (Goodwin et al., 2012). However, this decline could have been due to an increase in fishing pressure. Sensitivity assessment. The distribution of Palinurus elephas from Scotland to the Mediterranean suggests it would be resistant to a long-term change of 2°C in UK waters. It might increase in abundance in the northern UK (Hiscock et al., 2001). However, an increase in temperature may reduce egg production and larval recruitment. Furthermore, a short-term, acute, temperature change may also result in adult mortality, although wild adults might be expected to avoid thermal effluents. This suggests a resistance score of ‘Low’ with ‘Medium’ confidence. The resilience of the species is probably ‘Low’ and sensitivity is assessed as ‘High’. | LowHelp | LowHelp | HighHelp |
Temperature decrease (local) [Show more]Temperature decrease (local)Benchmark. A 5°C decrease in temperature for one month, or 2°C for one year. Further detail EvidenceDecreases in temperature may result in a reduction of population distribution in the British Isles. Crisp (1964a) reported that Palinurus elephas (studied as Palinurus vulgaris) held in the aquarium of the Marine Biological Station on the Isle of Man died during the severe winter of 1962-63. The water in the aquarium was supplied directly from Port Erin Bay and dropped to 3.5°C (the coldest since records began 60 years previously). In culture, lowering the water temperature of the cultures from 18-20 to 15-17°C did not affect the survival of the Palinurus elephas phyllosomas (Kittaka et al., 2001). Sensitivity assessment. Therefore, an acute change in temperature (a reduction in temperature of 5°C for 1 month), may result in local mortality, although in the wild adults might be expected to avoid thermal effluents or migrate to deeper waters. Hence, a resistance of ‘Medium’ is suggested. The resilience of the species is probably ‘Low’ and sensitivity is assessed as ‘Medium’. | MediumHelp | LowHelp | MediumHelp |
Salinity increase (local) [Show more]Salinity increase (local)Benchmark. A increase in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidencePalinurus elephas inhabits oceanic waters at 35 ppt salinity. It is unlikely to be subjected to further increases in salinity, except due to localised hypersaline effluents. Adults might be expected to avoid such effluents. However, there is 'No evidence' on which to base an assessment. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Salinity decrease (local) [Show more]Salinity decrease (local)Benchmark. A decrease in one MNCR salinity category above the usual range of the biotope or habitat. Further detail EvidenceIn the laboratory, Palinurus elephas survived direct transfer from seawater (38 ppt) to 27 ppt. The return to prior haemolymph osmolality took 24h. Direct transfer to lower salinities (20 ppt) resulted in mortality (Lucu et al., 2000). However, gradual acclimation showed a tolerance to more extreme dilution at 20 ppt. (decrease of 2 ppt every two days) suggesting an acclimation ability to a steady decrease in salinity (Lucu et al., 2000). Palinurus elephas were only kept for acclimation for two weeks so that long-term effects were unclear. In the wild, reduced salinity conditions would probably cause migration to areas of ‘normal’ salinity. This abnormal migration may interfere with feeding and reproduction. Individuals are only likely to be vulnerable during inshore migration for egg laying (see life history) as these are areas most likely to experience low salinity. Palinurus elephas is more vulnerable to stress during mating and egg production (Ansell & Robb, 1977). Sensitivity assessment. Therefore, the resistance of Palinurus elephas to a decrease in one MNCR salinity (‘full’ to ‘reduced’) category is recorded as ‘Medium’ but with ‘Low’ confidence. The resilience of the species is probably ‘Low’ and sensitivity is assessed as ‘Medium’. | MediumHelp | LowHelp | MediumHelp |
Water flow (tidal current) changes (local) [Show more]Water flow (tidal current) changes (local)Benchmark. A change in peak mean spring bed flow velocity of between 0.1 m/s to 0.2 m/s for more than one year. Further detail EvidencePalinurus elephas has been found in habitats with water flows ranging from very weak to very strong (JNCC, 1999). Most populations are found in regions dominated by strong ocean currents or gyres (Groeneveld et al., 2013). It is not likely to inhabit sedimentary areas, although it can forage in them. They are likely to prefer areas of good water flow or wave mediated flow, as both can keep the area clear of sediment. Therefore, an increase in water flow at the benchmark level is probably insignificant as the species occurs in areas of strong flow. A significant decrease, in the absence of wave action, could result in siltation, smothering and change in the habitat, from which it would have to move/relocate. However, a change at the benchmark level (0.1-0.2 m/s) is probably insignificant in strong flow (>1.5 m/s). Therefore, resistance and, hence, resilience are assessed as 'High' and this species is assessed as ‘Not Sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Emergence regime changes [Show more]Emergence regime changesBenchmark. 1) A change in the time covered or not covered by the sea for a period of ≥1 year or 2) an increase in relative sea level or decrease in high water level for ≥1 year. Further detail EvidencePalinurus elephas is found sublittoral and so will not be affected by a change in emergence. ‘Not relevant’ is recorded. However, it should be noted that Palinurus elephas are stored alive out-of-water for up to two days for marketing. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Wave exposure changes (local) [Show more]Wave exposure changes (local)Benchmark. A change in near shore significant wave height of >3% but <5% for more than one year. Further detail EvidencePalinurus elephas is recorded from wave exposed, rocky habitats. Therefore, a change in significant wave height of 3-5% is probably not significant in these habitats. Hence, resistance and resilience are 'High' and sensitivity is assessed as ‘Not sensitive’ at the benchmark level. | HighHelp | HighHelp | Not sensitiveHelp |
Chemical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Transition elements & organo-metal contamination [Show more]Transition elements & organo-metal contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceNot assessed | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Hydrocarbon & PAH contamination [Show more]Hydrocarbon & PAH contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceNot assessed | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Synthetic compound contamination [Show more]Synthetic compound contaminationBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceNot assessed | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Radionuclide contamination [Show more]Radionuclide contaminationBenchmark. An increase in 10µGy/h above background levels. Further detail EvidenceNo evidence | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of other substances [Show more]Introduction of other substancesBenchmark. Exposure of marine species or habitat to one or more relevant contaminants via uncontrolled releases or incidental spills. Further detail EvidenceNot assessed | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
De-oxygenation [Show more]De-oxygenationBenchmark. Exposure to dissolved oxygen concentration of less than or equal to 2 mg/l for one week (a change from WFD poor status to bad status). Further detail EvidenceCrustaceans were reported to be the most sensitive benthic organism to hypoxia based on a meta-analysis (Vaquer-Sunyer & Duarte, 2008). Most crustaceans would be lost (die) before the content of the water reached the threshold of 2 mg O2/litre (Vaquer-Sunyer & Duarte, 2008). Ansell and Robb (1977) recorded Palinurus elephas as being not very tolerant to oxygen depletion, as a malfunction in tank circulation in their 800l aquarium resulted in immediate death for many individuals, although their work was based on a small sample size and the oxygen levels involved were not reported. Sensitivity Assessment. The available evidence suggests that most crustaceans have a low resistance to hypoxia. Palinurus elephas is a mobile species and may be able to avoid localised hypoxia, depending on the extent and duration of the affected area so that only a proportion of the population may be affected. Resistance is therefore assessed as 'Low' but with 'low' confidence. Resilience is probably 'Low' and sensitivity is assessed as 'High'. | LowHelp | LowHelp | HighHelp |
Nutrient enrichment [Show more]Nutrient enrichmentBenchmark. Compliance with WFD criteria for good status. Further detail EvidenceNutrient enrichment from pollution and other sources can lead to an increase in algal blooms. In turn, this can lead to periods of deoxygenation (see deoxygenation pressure). No evidence specific to this species was found. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Organic enrichment [Show more]Organic enrichmentBenchmark. A deposit of 100 gC/m2/yr. Further detail EvidenceOrganic enrichment can lead to nutrient enrichment and deoxygenation. Organic enrichment encourages the productivity of suspension and deposit feeding detrivores and allows species to colonize the affected area to take advantage of the enhanced food (see removal on non-target species). However, 'No evidence' specific to this species was found. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Physical Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Physical loss (to land or freshwater habitat) [Show more]Physical loss (to land or freshwater habitat)Benchmark. A permanent loss of existing saline habitat within the site. Further detail EvidenceAll marine benthic species are considered to have a resistance of ‘None’ to this pressure and to be unable to recover from a permanent loss of habitat (resilience is ‘Very Low’). Sensitivity within the direct spatial footprint of this pressure is, therefore ’High’. Although no specific evidence is described confidence in this assessment is ’High’, due to the incontrovertible nature of this pressure. | NoneHelp | Very LowHelp | HighHelp |
Physical change (to another seabed type) [Show more]Physical change (to another seabed type)Benchmark. Permanent change from sedimentary or soft rock substrata to hard rock or artificial substrata or vice-versa. Further detail EvidenceThe selection of appropriate substrata providing adequate shelter is key in determining survival, especially in post-settlement stage (Diaz et al., 2011). There is a huge reliance on structured refuges such as crevices and caves (Gristina et al., 2011) due to a lack of ability to create their own shelters. This can create a bottleneck for recruitment especially in areas of high predation from predators such as Octopus vulgaris. Sheltering is one of their main and most effective defence mechanisms (Buscaino et al., 2011). The abundance of recently settled Palinurus elephas was found up to 10x lower in metamorphic substratum areas compared adjacent ‘optimal’ limestone area (Diaz et al., 2005). This is due to a preference for a softer sedimentary rock with more crevices. Therefore, a change from hard rock or soft rock to sediment would result in loss of suitable habitat and loss of the species from the affected area. Hence, resistance is assessed as ‘None’. The change is defined as permanent so that resilience is assessed as ‘Very low’ and sensitivity is assessed as ‘High’. Although no specific evidence is described, confidence in this assessment is ‘High’ due to the incontrovertible nature of this pressure.
| NoneHelp | Very LowHelp | HighHelp |
Physical change (to another sediment type) [Show more]Physical change (to another sediment type)Benchmark. Permanent change in one Folk class (based on UK SeaMap simplified classification). Further detail EvidencePalinurus elephas inhabits hard rock substratum. This benchmark only applies to sedimentary infauna. Therefore ‘Not relevant’ is recorded. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Habitat structure changes - removal of substratum (extraction) [Show more]Habitat structure changes - removal of substratum (extraction)Benchmark. The extraction of substratum to 30 cm (where substratum includes sediments and soft rock but excludes hard bedrock). Further detail EvidenceThis benchmark only applies to soft rock and sediment. Therefore ‘Not relevant’ is recorded. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Abrasion / disturbance of the surface of the substratum or seabed [Show more]Abrasion / disturbance of the surface of the substratum or seabedBenchmark. Damage to surface features (e.g. species and physical structures within the habitat). Further detail EvidenceCrawfish need their antennae to detect predators. Legs are also important for locomotion as returning to their shelter is a primary defence (Buscaino et al., 2011). When threatened by an octopus in the open, Palinurus elephas attempt to escape by using tail flips, darting backwards away from the attacker (Buscaino et al., 2011). Palinurus elephas has a tough exoskeleton. At the benchmark level, some damage may occur, for example, broken legs, but is unlikely to cause death in the majority of the population. The impact on the habitat by the global tangle net fishery was scored as very low risk (Tully et al., 2003). Trammel nets have shown to harbour relatively little impact on the seabed compared to other fishing activities such as trawling. Discarded specimens of Palinurus elephas in the observed trammel nets were almost exclusively damaged individuals. However, the gear is designed so that, Palinurus elephas arrive at the merchant’s premises alive (Quetglas et al., 2004). Net fisheries can result in mortality depending on the length of time Palinurus elephas is left in the nets and the levels of damage through contact with the net. As the nets are not very selective, the can result in catches of undersized individuals. If nets are not cleared regularly, this can result in juvenile mortalities (Mouat & Shelmerdine, 2012). There are potential localised physical effects of traps on sediment when hauling and/or deploying. Fragile benthic species may potentially be damaged during both deployment and hauling. Discarded pots, like nets, can ghost fist, catching both commercial and non-commercial species (European economic interest groups, 2014). Adult shelter in crevices and overhangs in the rocky substratum during the day. Therefore, they are likely to be protected from passing gears during the day but vulnerable at night when they are foraging. Abrasion that reduces the cover of turf-forming species may reduce the survival of juveniles. Sensitivity assessment. As individuals tend to reside in shelters during the day, they are unlikely to be caught or damaged by fishing gear and receive protection from passing gears. However, the population may be vulnerable to abrasion at night. Similarly, the juvenile habitat may be damaged by abrasion. However, the portion of the population affected will depend on the time of day, season, and geology of the habitat. Therefore, a resistance of ‘Medium’ has been suggested with ‘Low’ confidence. Resilience is probably ‘Low’ and sensitivity is assessed as ‘Medium’. | MediumHelp | LowHelp | MediumHelp |
Penetration or disturbance of the substratum subsurface [Show more]Penetration or disturbance of the substratum subsurfaceBenchmark. Damage to sub-surface features (e.g. species and physical structures within the habitat). Further detail EvidencePenetration is not relevant for hard substratum dwelling species. Therefore ‘Not relevant’. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Changes in suspended solids (water clarity) [Show more]Changes in suspended solids (water clarity)Benchmark. A change in one rank on the WFD (Water Framework Directive) scale e.g. from clear to intermediate for one year. Further detail EvidenceAn increase in the amount of suspended sediment is unlikely to affect Palinurus elephas directly. Palinurus elephas appear to select shelters with the principal purpose of reducing the risk of attack by minimising visual detection. They prefer dens on shaded, sub-vertical substratum. This not only avoids daylight but reduces siltation rate in their holes (Gristina et al., 2009). However, over the course of the benchmark, and depending on local hydrographic conditions, siltation may occur on the rocky substratum on which this species prefers. An increase in the amount of fine particulates, although unlikely to significantly change the nature of the substratum over the benchmark period, may alter the proportion of different prey items available to the crawfish. However, they can remain in their shelters with no feeding efforts for a week before their moult (Groeneveld et al., 2013) and may be tolerant of periods of starvation. Sensitivity assessment. Since Palinurus elephas are active omnivores and undergo periods of fasting, a change in suspended sediment is unlikely to reduce total ingestion over the benchmark period. Therefore resistance has been assessed as ‘Medium’ with ‘Low’ confidence. The resilience is probably ‘Low’ and sensitivity is assessed as ‘Medium’. | MediumHelp | LowHelp | MediumHelp |
Smothering and siltation rate changes (light) [Show more]Smothering and siltation rate changes (light)Benchmark. ‘Light’ deposition of up to 5 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceThe species is quite large and mobile. Smothering by 5 cm of sediment is unlikely to adversely affect adult Palinurus elephas. Palinurus elephas appear to select shelters with the principal purpose of reducing the risk of attack by minimising visual detection. They prefer dens on shaded, sub-vertical substratum. This not only avoids daylight but reduces siltation rate changes in their holes (Gristina et al., 2009). Crawfish vulnerability decreases significantly on vertical substrata. They prefer semi-circular dens enabling them to restrict the entrance and resit extraction. A light deposition of sediment is unlikely to cause an adverse effect due to the position of the preferred shelter and size of the organism. Therefore, a resistance score of ‘High’ has been suggested with ‘Medium’ confidence. Resilience is assessed as ‘High and sensitivity is assessed as ‘Not sensitive’ to light deposition of sediment.
| HighHelp | HighHelp | Not sensitiveHelp |
Smothering and siltation rate changes (heavy) [Show more]Smothering and siltation rate changes (heavy)Benchmark. ‘Heavy’ deposition of up to 30 cm of fine material added to the seabed in a single discrete event. Further detail EvidenceA heavy deposition could lead to an obstruction of the shelter entrance reducing the ability of Palinurus elephas to enter to leave their crevice. The deposition could also cause an indirect effect on the crawfish by smothering potential prey. Therefore, resistance is assessed as 'Low' for this benchmark within the affected area. However, confidence is ‘Low’. The resilience of the species is probably ‘Low’ and sensitivity is assessed as ‘High’. | LowHelp | LowHelp | HighHelp |
Litter [Show more]LitterBenchmark. The introduction of man-made objects able to cause physical harm (surface, water column, seafloor or strandline). Further detail Evidence'Not assessed'. However, it is noted that Palinurus elephas will use shipwrecks and other man-made substrata that have found its way to the seabed as shelter. | Not Assessed (NA)Help | Not assessed (NA)Help | Not assessed (NA)Help |
Electromagnetic changes [Show more]Electromagnetic changesBenchmark. A local electric field of 1 V/m or a local magnetic field of 10 µT. Further detail Evidence'No evidence' | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Underwater noise changes [Show more]Underwater noise changesBenchmark. MSFD indicator levels (SEL or peak SPL) exceeded for 20% of days in a calendar year. Further detail EvidenceFemale Palinurus elephas stridulate in order to attract males (see Reproduction). In controlled laboratory conditions, female locomotive behaviour increased under playbacks of male “rasps” (Filiciotto et al., 2014). The acoustic signals emitted by Palinurus elephas is within the range of ultrasonic frequencies (20-55 kHz), (Edmonds et al., 2016). Noise disturbance may interfere with the production or reception of these mating signals. Specimens of males and female Palinurus elephas in the same laboratory were exposed to recordings of ‘boat’ noise (ca 75-125 dB) from a nearby harbour in Italy, frequented by a range of vessels. Signals and ‘rasps’ were emitted in 'control' conditions. It was suggested that these provide interaction with group members in this gregarious species (Filiciotto et al., 2015). The rasps are acoustic emissions caused by tail whips that cause a movement of antennae. These can be used for anti-predatory behaviour. In the presence of an octopus, individually and in groups, more rasps could cover a wide range of ocean (Buscaino et al., 2011(b)). Groups behaviours are thought to provide advantages due to shared shelter, defence and detection of predators (Filiciotto et al., 2015). In the laboratory, artificial noise caused a significant increase in locomotor behaviour, which could deplete their energy reserves. Artificial noise also resulted in a loss of group behaviours in favour of individual behaviours, which could increase their vulnerability to predation (Filiciotto et al., 2014) or interfere with mating. In addition, noise changed haemolymphatic parameters (Filiciotto et al., 2014). Haemolymphatic biochemistry is a bioindicator for stressful conditions and poor health in crustaceans (Filiciotto et al., 2014). Artificial noise resulted in haemolymph parameters typical of stress and reduced immune response and increased risk of disease (Filiciotto et al., 2014). After experiencing shipping noise in a tank HSP27 (heat shock protein no. 27) increased three fold and the levels of haemocytes decreased both reflecting a stress response (Celi et al., 2015). Sensitivity assessment. The noise treatments used in the above studies do not compare easily with the benchmark leave of pressure. However, the available evidence suggests that artificial noise from ’boats’ in a harbour could interfere with, group behaviour, mating, and induce stress in Palinurus elephas, as in doing so increase their risk of predation and disease and reduce reproductive success. Therefore, resistance has been assessed as ‘Medium’ as a precaution but with ‘Low’ confidence as further study is required. Lack of evidence for a permanent effect of exposure to noise means the resilience is probably ‘High’. Hence, the sensitivity of this species to noise pollution is assessed as ‘Low’.
| MediumHelp | HighHelp | LowHelp |
Introduction of light or shading [Show more]Introduction of light or shadingBenchmark. A change in incident light via anthropogenic means. Further detail EvidencePalinurus elephas are a nocturnal species and prefer crevices on a 90°, vertical, angle to reduce light entering the shelter. Palinurus elephas prey nocturnally and, therefore, rely on the lack of light to reduce detection and predation. The abundance of Palinurus elephas decreased significantly under a full moon suggesting a high sensitivity for light (Goñi et al., 2001). Artificial light tends to mirror the moonlight (Groeneveld et al., 2013). There is a potential for artificial light placed on a habitat to interfere with the nocturnal feeding and movement activities leading to a suggested resistance score of ‘Medium’ but with ‘Low’ confidence. Resistance is probably ‘Low’ and sensitivity is ‘Medium’. | MediumHelp | LowHelp | MediumHelp |
Barrier to species movement [Show more]Barrier to species movementBenchmark. A permanent or temporary barrier to species movement over ≥50% of water body width or a 10% change in tidal excursion. Further detail EvidenceIncreasing evidence is showing a reduction in population gene flow. The restriction is most likely caused by oceanographic factors such as the Gulf Stream and mesoscale processes from the Atlantic Ocean restricting the larval distribution (see life history). Despite being a mobile species, they do not move around a lot and are mostly sedentary and population subdivision results from lack of gene flow via larval dispersal. This pressure is considered applicable to mobile species, e.g. fish and marine mammals rather than seabed habitats. Physical and hydrographic barriers may limit the dispersal of larvae but larval dispersal is not considered under the pressure definition and benchmark. Therefore, this pressure is described as ‘Not relevant’.
| Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Death or injury by collision [Show more]Death or injury by collisionBenchmark. Injury or mortality from collisions of biota with both static or moving structures due to 0.1% of tidal volume on an average tide, passing through an artificial structure. Further detail EvidenceNot relevant to seabed habitats and benthic species. NB. Collision by grounding vessels is addressed under ‘surface abrasion’. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Visual disturbance [Show more]Visual disturbanceBenchmark. The daily duration of transient visual cues exceeds 10% of the period of site occupancy by the feature. Further detail EvidenceDecapod crustaceans have compound eyes and, therefore, have quite good vision and are likely to respond to movement in order to avoid predators. However, it is unlikely that the species will be affected by visual disturbance at the benchmark level. Therefore 'Not relevant'. | Not relevant (NR)Help | Not relevant (NR)Help | Not relevant (NR)Help |
Biological Pressures
Use [show more] / [show less] to open/close text displayed
| Resistance | Resilience | Sensitivity | |
Genetic modification & translocation of indigenous species [Show more]Genetic modification & translocation of indigenous speciesBenchmark. Translocation of indigenous species or the introduction of genetically modified or genetically different populations of indigenous species that may result in changes in the genetic structure of local populations, hybridization, or change in community structure. Further detail EvidencePalinurus elephas is notoriously difficult to rear in aquaculture despite hatching at an advanced stage (see e.g. Kittaka & Ikegami, 1988) and attempts at culture have been unsuccessful. There is evidence for genetic differentiation between the populations from Britany and UK as well as between the Atlantic and Mediterranean populations. This difference is due to a limited gene flow and suggests a restriction on larval dispersal. However, there is 'No evidence' that the species has been translocated or genetically modified, although, individuals caught in western Scotland have been transferred to the St Abbs Marine Station for study (K. Hiscock, pers. comm.). | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction or spread of invasive non-indigenous species [Show more]Introduction or spread of invasive non-indigenous speciesBenchmark. The introduction of one or more invasive non-indigenous species (INIS). Further detail EvidenceThe red king crab has the potential to invade GB from the northern Atlantic, Norwegian coast. Paralithodes camtschaticus is a large, highly mobile, generalist predator. This invasive predator reduces biomass, especially in bivalves and barnacles. It also feeds on molluscs and echinoderms, same as Palinurus elephas (ICES, 2005). Like Palinurus elephas, it also has a twenty-year lifespan and pelagic larvae. Despite the potential impacts, Paralithodes camtschaticus has not yet been reported in the UK. Therefore, there is ‘No evidence’ currently on the effects of non-native species on Palinurus elephas. | No evidence (NEv)Help | Not relevant (NR)Help | No evidence (NEv)Help |
Introduction of microbial pathogens [Show more]Introduction of microbial pathogensBenchmark. The introduction of relevant microbial pathogens or metazoan disease vectors to an area where they are currently not present (e.g. Martelia refringens and Bonamia, Avian influenza virus, viral Haemorrhagic Septicaemia virus). Further detail EvidencePalinurus elephas is susceptible to crustacean shell disease, which is characterized by brown spots that erode away the exoskeleton. These lesions are often found to contain chitinoclastic bacteria. In addition, Alderman (1973) recorded the presence of a fungus bearing septate mycelium. Mortality of this individual occurred after six weeks. The fungus was found to have entered the body via ingestion and suggests that fungal related diseases are able to infect crawfish and appear to be less treatable than bacterial infections (Alderman, 1973). A total of 198 bacterial strains were identified amongst nine caught individuals. Despite this, no mortality was recorded over a period of 34 days and the bacteria appeared to be concentrated in the areas of infection (Mancuso et al., 2010). Vibrio infections can cause serious diseases in spiny lobsters. Few cases have been reported and these have been in fisheries (Shields, 2011). Peritrich and suctorian ciliates are often found on the external surface of embryos, which indicates mother is unhealthy, probably due to not feeding well (Shields, 2011). For both diseases, no mortality was recorded over the study period. Sensitivity assessment. The presence of diseases in the population will result in reduced health and viability of a proportion of the population. However, no evidence of large-scale mortality was found so resistance is assessed as ‘High’. Therefore, resilience is probably ‘High’ and sensitivity is assessed as ‘Not sensitive’. | HighHelp | HighHelp | Not sensitiveHelp |
Removal of target species [Show more]Removal of target speciesBenchmark. Removal of species targeted by fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidencePalinurus elephas is one of the most commercially important spiny lobster species in the Mediterranean and northeast Atlantic (Goñi, 2005). This species is taken both as a targeted species and as a by-catch from other fisheries. Intensive potting (creeling), diving and tangle or trammel netting for Palinurus elephas has contributed to a very substantial decline in population size since the 1970's For instance, the Welsh pot-hauled crawfish fishery fell from 55,000 kg in 1979 to less than 500kg in 1995 (Hunter, 1999). It is also highly exploited in the Mediterranean where a commercial decline of 30-50% has been observed over the last 30 years (Gonulal, 2015). During fishing, intermediate survival was high for undersized lobsters in nets and traps allowing a safe return. However, after seven days left in the nets or traps survival was reduced to 64% (Catanese et al., 2018). Despite the effort for regulation and policing, boats may have up to 10,000 m of trammel nets which they haul throughout the season and may leave to fish for three or more days (Goñi et al., 2003). The use of plastic traps usually results in little or no mortality of Palinurus elephas. However, when compared to collapsible traps, the mortality of Palinurus elephas in these traps was 1/3 of the total catch (Amengual-Ramis et al., 2016). It has been suggested that mortality from fishing and due to bycatch is because of scavenging crustaceans and/or net damage. This emphasises the danger of leaving crustaceans including Palinurus elephas in fishing gear for long periods of time (Bord Iascaigh Mhara, 2011). In recent studies from the western Mediterranean, efforts have been made to introduce good practice for releasing undersized individuals in good condition (Franco, 2004). Measures are in place to reduce the impact of fisheries. They include local bye-laws that prohibit the landing of berried lobsters and that set size limits for marketable lobsters (often 110 mm CL). Larger females produce five times more eggs and larger larvae (Goñi et al., 2003) and exploitation usually targets larger individuals with newly mature females accounting for only 1% of egg production. Sensitivity assessment. Resistance is ‘None’ with ‘Medium’ confidence due to the increase if efficient fishery catching methods. The collapse of populations after over-fishing and the very long time taken for recovery in south-west Britain at least is a ‘lesson’ for recovery potential and resilience is ‘Very low’. This species is, therefore, predicted to have a ‘High’ sensitivity to removal. | NoneHelp | Very LowHelp | HighHelp |
Removal of non-target species [Show more]Removal of non-target speciesBenchmark. Removal of features or incidental non-targeted catch (by-catch) through targeted fishery, shellfishery or harvesting at a commercial or recreational scale. Further detail EvidencePalinurus elephas rely on structured refuges such as crevices and caves (Gristina et al., 2011). Juveniles benefit from the burrows left by other species, e.g. in the Mediterranean the burrows in soft rock made by date mussels are an important habitat (Diaz et al., 2001). This can create a bottleneck for recruitment especially in areas of high predation from octopus due to increase completion for shelters. Molluscs and sea urchins are the most important prey in their diet (Philips, 2013). This means a removal of these species could have an indirect effect on the survival of Palinurus elephas. Palinurus elephas is considered to be by-catch of some 100 finfish fisheries. The genera Homarus, Cancer, Palinurus and Necora can all be caught using the same traps (Tully et al., 2003). In Wales, in 2003, 65% of crawfish landed were caught as a result of bycatch from lobster fisheries (Perry, 2015). In Scotland, Palinurus elephas are caught as a bycatch in the bottom-set tangle net fishery for monkfish (Chapman, 2006). Moving from the use of pots to trammel nets has increased the vulnerability of Palinurus elephas as a bycatch species. This is because both trammel and tangling nets are more efficient in capturing different individuals due to a lack of selectivity. Therefore, many species of different sizes are caught including Palinurus elephas even if it is not the target species (Trindade-Gonçalves, 2010). An investigation into what happens to the individuals caught via by-catch has shown that 75% of Palinurus elephas caught were sold despite not being targeted (Batista et al., 2009). This means that Palinurus elephas is not only vulnerable to target fishing but also has the potential to be caught alongside other fish species in nets and crustaceans in pots. Sensitivity assessment. Resistance to the removal of non-target species is ‘Low’ due to the potential reduction in prey and the vulnerability of Palinurus elephas to be caught as by-catch. Resilience is probably 'Low' and sensitivity is, therefore, assessed as 'High'. | LowHelp | LowHelp | HighHelp |
Importance review
Policy/legislation
| Designation | Support |
|---|---|
| UK Biodiversity Action Plan Priority | Yes |
| Species of principal importance (England) | Yes |
| Species of principal importance (Wales) | Yes |
| Features of Conservation Importance (England & Wales) | Yes |
| Priority Marine Features (Scotland) | Yes |
Status
| National (GB) importance | Not rare or scarce | Global red list (IUCN) category | Vulnerable (VU) |
Non-native
| Parameter | Data |
|---|---|
| Native | Native |
| Origin | Not relevant |
| Date Arrived | - |
Importance information
Palinurus elephas is the most valued large decapod throughout its range, potentially due to it being more accessible compared to other species, such as Palinurus mauritanicus (Babbucci et al., 2010). It has a high unit value of (40-120 €/Kg). High levels of fishing have reduced the genetic diversity of the populations (Tufto & Hindar, 2003). In the North East Atlantic, it is a by-catch species where fishing has depleted its populations (Latrouite & Noel, 1997, cited in Goñi et al., 2003).
There are peripheral fisheries off the south and west coasts of England, Wales, Scotland, and Ireland. Landings from Scotland in 1998 amounted to 15.8 metric tonnes, mostly from the southwest around Barra and South Uist. A considerable fishery existed off the Cornish coast in the 1970's but the population was over-exploited and nowadays exists at a much lower intensity. Total landings of Palinurus elephas in France have seen a downward trend with landings at 137, 128, 112, 104, 90, 44, 58, 59, 65 and 46 mt for the years 1993-2002 although again, no indication of effort is given. Declines in number are documented for crawfish fisheries elsewhere along the European Atlantic coast e.g. Ireland, Spain and Portugal. (Lock, 2011).
The collapse of populations in south-west Britain because of overfishing in the late 1960s and through the 1970s at least was not reversed until about 2014 when large settlements and increasing populations of young crawfish were widely reported. This long period before apparent recovery is seen as a ‘lesson’ that recovery of over-exploited populations can take a long time and that such fisheries need to be well-managed (K. Hiscock, pers. comm.)
Protection. No fishing quotas are currently applied. In most fishing areas, fishing decreases or ceases during the breeding period. It is not permitted to catch berried females and the mesh size of the nets on fishing boats must be regulated (Quetglas et al., 2004). Non-quota species, such a Palinurus elephas, are more likely to be fished by inshore vessels. This means they are subject to less monitoring control and surveillance (Jones, 2011).
Palinurus elephas is considered to be a bycatch of some 100 finfish fisheries. By-catch risk for this fishery has been assessed as a moderate risk (Quetglas et al., 2004). Mitigating measures are in place in vessels > 12 m. The habitat impact of the tangle net fishery has been scored as very low risk (Quetglas et al., 2004). Trammel nets have been shown to harbour relatively little impact on the seabed compared to other fishing activities such as trawling. Anchoring of the nets has minimal impact on the sea floor. Despite this, they have the potential to be lost in bad weather, such as storms, and become ghost nets. In order to buffer the impact on recruitment from overfishing it is important to maintain populations with a broad range of age and size classes (Diaz et al., 2016) (see population dynamics). Reintroduction of males and females in unfished areas, around Britain, may help to increase reproductive stock (Hiscock et al., 2011) but, subsequent to that conclusion, recovery has occurred without intervention. The closed-season management measure appears to be the most effective currently (IUCN, 2018). This entails a ban on fishing during the months of May-August in countries such as Spain and Portugal to protect the species during their more vulnerable period of mating. Crustacean fisheries in Greece have a closed season of four months, generally from September to December. There is also a ban on the capture of berried females Minimum landing sizes; 110 cm CL; Ireland, England, Wales and Scotland. 9.5 cm in France, 8.5 in Greece and 8 cm in Spain and Italy (Tully et al., 2003).
Goñi et al. (2001) studied a population of Palinurus elephas that had been afforded protection by the Columbretes Islands Marine Reserve (Mediterranean) for eight years. They reported that catch rates in the reserve were conspicuously higher than at two unprotected sites. Furthermore, catch rates at one of the fished areas increased significantly after a six-month closed season. The fishery that has developed at the boundaries of the reserves suggests that spill over takes place. In 2018 Seasearch, south and west Wales has increased publicity of the crawfish in order to record sightings and increase management efforts. The Manacles Marine Conservation Zone (MCZ) in south Cornwall has listed Palinurus elephas as one of their key species of protection (Bolton, 2018).
Aquaculture. Palinurus elephas are, in theory, attractive for culture (Ceccaldi & Latrouite, 1994). However, Palinurus elephas is notoriously difficult to rear in the laboratory despite hatching at an advanced stage (see life history, Kittaka & Ikegami, 1988) and attempts at culture have been unsuccessful.
Bibliography
Alderman, D.J., 1973. Fungal infection of crawfish (Palinurus elephas) exoskeleton Transactions of the British Mycological Society, 61, 595-597
Amengual-Ramis, J.F., Vázquez-Archdale, M., Cánovas-Pérez, C. & Morales-Nin, B., 2016. The artisanal fishery of the spiny lobster Palinurus elephas in Cabrera National Park, Spain: Comparative study on traditional and modern traps with trammel nets. Fisheries Research, 179, 23-32.
Ansell, A. D. & Robb, L., 1977. The spiny lobster Palinurus elephas in Scottish waters. Marine Biology, 43, 63-70.
Babbucci, M., Buccoli, S., Cau, A., Cannas, R., Goñi, R., Díaz, D., Marcato, S., Zane, L. & Patarnello, T., 2010. Population structure, demographic history, and selective processes: Contrasting evidences from mitochondrial and nuclear markers in the European spiny lobster Palinurus elephas (Fabricius, 1787). Molecular Phylogenetics and Evolution, 56 1040-1050.
Batista, M.I., Teixeira, C.M. & Cabral, H.N., 2009. Catches of target species and bycatches of an artisanal fishery: The case study of a trammel net fishery in the Portuguese coast. Fisheries Research, 100, 167-177.
Bevacqua, D., Melià, P., Follesa, M.C., De Leo, G.A., Gatto, M. & Cau, A., 2011. Erratum to “Body growth and mortality of the spiny lobster Palinurus elephas within and outside a small marine protected area”. Fisheries Research, 108, 404.
Bolton, C., 2018. Return of the crawfish? Porcupine Bulletin Number 9 – Spring 2018
Borges, P., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., Santos, R., Silva, L., Vieira, P. & Vieira, V., 2010. A List of the Terrestrial and Marine Biota From the Azores. Princípia, Cascais, 432 pp.
Bruce, J.R., Colman, J.S. & Jones, N.S., 1963. Marine fauna of the Isle of Man. Liverpool: Liverpool University Press.
Buscaino, G., Filiciotto, F., Gristina, M., Bellante, A., Buffa, G., Di Stefano, V., Maccarrone, V., Tranchida, G., Buscaino, C. & Mazzola, S., 2011. Acoustic behaviour of the European spiny lobster Palinurus elephas. Marine Ecology Progress Series, 441, 177-184.
Buscaino, G., Filiciotto, F., Gristina, M., Buffa, G., Bellante, A., Maccarrone, V., Patti, B. & Mazzola, S., 2011. Defensive strategies of European spiny lobster Palinurus elephas during predator attack. Marine Ecology Progress Series, 423, 143-154.
Campillo, A. & Amadei, J., 1978. Première donné es biologiques sur la langouste de corse, Palinurus elephas, Fabricius. Revue des travaux de l'institut des pêches maritimes, Paris, 42, 343-373.
Catanese, G., Hinz, H., Gil, M.d.M., Palmer, M., Breen, M., Mira, A., Pastor, E., Grau, A., Campos-Candela, A., Koleva, E., Grau, A.M. & Morales-Nin, B., 2018. Comparing the catch composition, profitability and discard survival from different trammel net designs targeting common spiny lobster (Palinurus elephas) in a Mediterranean fishery. PeerJ, 6, e4707.
Catanese, G., Hinz, H., Gil, M.d.M., Palmer, M., Breen, M., Mira, A., Pastor, E., Grau, A., Campos-Candela, A., Koleva, E., Grau, A.M. & Morales-Nin, B., 2018. Comparing the catch composition, profitability and discard survival from different trammel net designs targeting common spiny lobster (Palinurus elephas) in a Mediterranean fishery. PeerJ, 6, e4707.
Ceccaldi, H.J. & Latrouite, D., 1994. The French fisheries for the European spiny lobster Palinurus elephas. Oxford: Fishing News Books.
Celi, M., Filiciotto, F., Vazzana, M., Arizza, V., Maccarrone, V., Ceraulo, M., Mazzola, S. & Buscaino, G., 2015. Shipping noise affecting immune responses of European spiny lobster Palinurus elephas (Fabricius, 1787). Canadian Journal of Zoology, 93, 113-121
Chapman, C.J., 2006. Coastal shellfish resources and fisheries in SEA7 Strategic environmental Assessment – SEA7. Technical Report for Department of Trade & Industry Geotek Ltd and Hartley Anderson Ltd, 76 pp.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Cunningham, J.T., 1891. On the Development of Palinurus vulgaris, the Rock Lobster or Sea Crayfish. Journal of the Marine Biological Association of the United Kingdom, 2 (2), 141-150. DOI 10.1017/S0025315400003866
Díaz, D., Mallol Martínez, S., Parma, A. & Goñi, R., 2016. A 25-year marine reserve as proxy for the unfished condition of an exploited species. Biological Conservation, 203, 97-107
Díaz, D., Mallol, S., Parma, A.M. & Goñi, R., 2011. Decadal trend in lobster reproductive output from a temperate marine protected area. Marine Ecology Progress Series, 433, 149-157.
Davies, G., 2016. The crawfish divers of Cornwall. Geoff Davies, Hayle. pp. 133
Diaz, D., Mari, M., Abello, P. & Demestre, M., 2001. Settlement and juvenile habitat of the European spiny lobster Palinurus elephas (Crustacea: Decapoda: Palinuridae) in the western Mediterranean Sea. Scientia Marina, 65, 347-256.
Edmonds, N.J., Firmin, C.J., Goldsmith, D., Faulkner, R.C. & Wood, D.T., 2016. A review of crustacean sensitivity to high amplitude underwater noise: Data needs for effective risk assessment in relation to UK commercial species. Marine Pollution Bulletin, 108, 5-11.
Elphie, H., Raquel, G., David, D. & Serge, P., 2012. Detecting immigrants in a highly genetically homogeneous spiny lobster population (Palinurus elephas) in the northwest Mediterranean Sea. Ecology and evolution, 2, 2387-2396.
FAO (Food and Agriculture Organization of the United Nations), 2002. Fisheries Statistics. Capture Production. Yearbook. Volume 94/1.
Filiciotto, F., Vazzana, M., Celi, M., Maccarrone, V., Ceraulo, M., Buffa, G., Stefano, V.D., Mazzola, S. & Buscaino, G., 2014. Behavioural and biochemical stress responses of Palinurus elephas after exposure to boat noise pollution in tank. Marine Pollution Bulletin, 84, 104-114.
Follesa, M., Cannas, R., Cau, A., Cuccu, D., Gastoni, A., Ortu, A., Pedoni, C., Porcu, C. & Cau, A., 2011. Spillover effects of a Mediterranean marine protected area on the European spiny lobster Palinurus elephas (Fabricius, 1787) resource. Aquatic Conservation Marine and Freshwater Ecosystems, 21, 564-572.
Follesa, M., Cannas, R., Cau, A., Cuccu, D., Mulas, A., Porcu, C., Saba, S. & Cau, A., 2014. Homing and orientation of Palinurus elephas (Fabricius) in three no-take areas of the central-western Mediterranean: Implications for marine reserve design. Marine and Freshwater Research, 66, 1-9
Follesa, M., Cuccu, D., Cannas, R., Sabatini, A. & Cau, A., 2007. Emigration and retention of Palinurus elephas (Fabricius, 1787) in a central western Mediterranean marine protected area. 71, 279-285.
Follesa, M., Cuccu, D., Cannas, R., Sabatini, A., Maria Deiana, A. & Cau, A., 2009. Movement patterns of the spiny lobster Palinurus elephas (Fabricius, 1787) from a central western Mediterranean protected area. Scientia Marina, 73, 499-506.
Follesa, M.C., Cuccu, D., Cannas, R. & Cau, A., 2007. On the growth of the European spiny lobster, Palinurus elephas from Sardinian waters (central western Mediterranean Sea). New Zealand Journal of Marine and Freshwater Research, 41, 377-383.
Franco, B., 2004. European Union Mediterranean Fisheries and exploited resources. Scientific, technical and economic committee for fisheries (STECF) 505 pp.
Galhardo, A.C., Serafim, P. & Castro, M., 2006. Aspects of the Biology and Fishery of the European Spiny Lobster (Palinurus Elephas) from the Southwest Coast of Portugal. Journal of Crustacean Biology, 26 601-609.
Giacalone, V.M., Barausse, A., Gristina, M., Pipitone, C., Visconti, V., Badalamenti, F. & D'Anna, G., 2015. Diel activity and short-distance movement pattern of the European spiny lobster, Palinurus elephas, acoustically tracked. Marine Ecology, 36, 389-399.
Giacalone, V.M., D'Anna, G., Pipitone, C. & Badalamenti, F., 2006. Movements and residence time of spiny lobsters, Palinurus elephas released in a marine protected area: an investigation by ultrasonic telemetry. Journal of the Marine Biological Association of the United Kingdom, 86, 1101-1106.
Gibson, F.A. & O'Riordan, C.E., 1965. Palinurus vulgaris (L.) the crawfish in Irish waters 1962. Rapports et Proces-verbaux des Reunions. Conseil Permanent International pour l'Exploration de la Mer, 156, 47-49.
Goñi , R. & Latrouite, D., 2005. Review of the biology, ecology and fisheries of Palinurus spp. species of European waters: Palinurus elephas (Fabricius, 1787) and Palinurus mauritanicus (Gruvell, 1911) Cahiers de biologie marine, 46, 127-142
Goñi, R, Reñones, O. & Quetglas, A., 2001. Dynamics of a protected Western Mediterranean population of the European spiny lobster Palinurus elephas (Fabricius, 1787) assessed by trap surveys. Marine and Freshwater Research, 52, 1577-1587.
Goñi, R., Quetglas, A. & Reñones, O., 2003. Size at maturity, fecundity and reproductive potential of a protected population of the spiny lobster Palinurus elephas (Fabricius, 1787) from the western Mediterranean. Marine Biology, 143, 583-592.
Gonulal, O., 2015. The Aegean Sea marine biodiversity, fisheries, conservation and governance.
Goodwin, C.E., Strain, E.M., Edwards, H., Bennett, S.C., Breen, J.P. & Picton, B.E., 2013. Effects of two decades of rising sea surface temperatures on sublittoral macrobenthos communities in Northern Ireland, UK. Marine Environmental Research, 85, 34-44.
Gristina, M., Fiorentino, F., Garofalo, G. & Badalamenti, F., 2009. Shelter preference in captive juveniles of European spiny lobster Palinurus elephas (Fabricius, 1787). Marine Biology, 156, 2097-2105.
Gristina, M., Sinopoli, M., Fiorentino, F., Garofalo, G. & Badalamenti, F., 2011. Shelter selection of the spiny lobster Palinurus elephas under different levels of Octopus vulgaris predation threat. Marine Biology, 158, 1331-1337
Groeneveld, C., Goñi, R., Díaz, D., 2013, Chapter 11: Palinurus species. Lobsters : Biology, Management, Aquaculture and Fisheries (ed. Phillips, 2013), pp. 326-348. Somerset, United Kingdom: John Wiley & Sons, Incorporated
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hepper, B. T., 1977. The fishery for crawfish, Palinurus elephas, off the coast of Cornwall. Journal of the Marine Biological Association of the United Kingdom, 57, 925-941.
Hiscock, K., Bayley, D., Pade, N., Cox, E. & Lacey, C., 2011. A recovery / conservation programme for marine species of conservation importance. A report to Natural England from the Marine Biological Association of the UK and SMRU Ltd. Natural England Commissioned Reports, Natural England, Peterborough, 65, 245
Holthuis, L.B., 1991. FAO Species Catalogue, vol. 13. Marine lobsters of the World. An annotated and illustrated catalogue of species of interest to fisheries known to date. Food and Aqriculture Organization of the United Nations, Rome, FAO Fisheries Synopsis, no. 125, 13, 292.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Hunter, E., 1999. Biology of the European spiny lobster, Palinurus elephas (Fabricius, 1787) (Decapoda, Palinuroidea) Crustaceana, 72, 545 - 565.
Hunter, E., Shackley, S. E. & Bennett, D. B., 1996. Recent studies on the crawfish Palinurus elephas in South Wales and Cornwall. Journal of the Marine Biological Association of the United Kingdom, 76, 963-983.
Ingle, R., 1997. Crayfishes, lobsters and crabs of Europe. An illustrated guide to common and traded species. London: Chapman and Hall.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Jones, J., & Lock, K., 2014. Distribution and abundance of Palinurus elephas in Pembrokeshire. Seasearch Report. Ross-on-Wye: The Marine Conservation Society.
Jones, J., 2012. Distribution and abundance of Palinurus elephas in North Pembrokeshire. Seasearch Report. Ross-on-Wye: The Marine Conservation Society.
Kelly-Fletcher, D. & Holt, D., 2015. Interim report on the Isles of Scilly IFCA Shellfish Tagging Project. Isles of Scilly Inshore Fisheries and Conservation Authority report no. IOS IFCA 2015/01 , pp. 19
Kittaka, J. & Abrunhosa, F.A., 1997. Characteristics of palinurids (Decapoda; Crustacea) in larval culture. Hydrobiologia, 358, 305-311.
Kittaka, J. & Ikegami, E., 1988. Culture of the Palinurid Palinurus elephas from egg stage to puerulus. Nippon Suisan Gakkaishi, 54, 1149-1154.
Kittaka, J., 1997. Application of ecosystem culture method for complete development of phyllosomas of spiny lobster. Aquaculture, 155, 319-331.
Kittaka, J., Kudo, R., Onoda, S., Kanemaru, K. & Mercer, J.P., 2001. Larval culture of the European spiny lobster Palinurus elephas. Marine and Freshwater Research, 52, 1439-1444.
Lebour, M.V., 1925. Young anglers in captivity and some of their enemies. A study in a plunger jar. Journal of the Marine Biological Association of the United Kingdom, 13, 721-734.
Lindley, J.A., 1987. Continuous plankton records: the geographical distribution and seasonal cycles of decapod crustacean larvae and pelagic post-larvae in the north-eastern Atlantic Ocean and the North Sea. Journal of the Marine Biological Association of the United Kingdom, 67, 145-167.
Lindley, J.A., 1998. Diversity, biomass and production of decapod crustacean larvae in a changing environment. Invertebrate Reproduction and Development, 33, 209-219.
Lindley, J.A., Williams, R. & Hunt, H.G., 1993. Anomalous seasonal cycles of decapod crustacean larvae in the North Sea plankton in an abnormally warm year. Journal of Experimental Marine Biology and Ecology, 172, 47-65.
Lock, K., 2011. Seasearch Wales Crawfish Palinurus elephas historical diver records. Seasearch and Wales Diversity Partnership Report. Ross-on-Wye: The Marine Conservation Society.
Lucu, Č., Devescovi, M., Skaramuca, B. & Kožul, V., 2000. Gill Na,K-ATPase in the spiny lobster Palinurus elephas and other marine osmoconformers: Adaptiveness of enzymes from osmoconformity to hyperregulation. Journal of Experimental Marine Biology and Ecology, 246, 163-178.
Mallol, S., Díaz, D., Sobrado, F. & Goñi, R., 2014. First V-Notching Experience of a Spiny Lobster: V-Notch Recovery and Impact on Health and Growth of Palinurus elephas. Journal of Crustacean Biology, 34, 25-30.
Mancuso, M., Costanzo, M.T., Maricchiolo, G., Gristina, M., Zaccone, R., Cuccu, D. & Genovese, L., 2010. Characterization of chitinolytic bacteria and histological aspects of Shell Disease Syndrome in European spiny lobsters (Palinurus elephas) (Fabricius 1787). Journal of Invertebrate Pathology, 104, 242-244.
Marin, J., 1997. La langouste rouge: biologie et exploitation. Pêche Maritime, 64, 105-113.
MBA (Marine Biological Association), 1957. Plymouth Marine Fauna. Plymouth: Marine Biological Association of the United Kingdom.
Mercer, J.P., 1973. Studies on spiny lobsters (Crustacea: Decapoda: Palinuridae) of the west coast of Ireland, with particular reference to Palinurus elephas, Fabricius 1787. PhD thesis, University College, Galway., PhD thesis, University College, Galway.
Mhara, B.I., 2011. Shellfish stocks and fisheries. Shellfish stocks & fisheries review. Marine Institute Foras na Mara, 90 pp.
Miller, R.J., 1990. Effectiveness of Crab and Lobster Traps. Canadian Journal of Fisheries and Aquatic Sciences, 47, 1228-1251.
Mouat, B. & Shelmerdine, R., 2012. Management measures for self propagated future recovery of crawfish, Palinurus elephas, in Welsh waters. Affiliation: Coutryside Council for Wales (CCW)
Noel, P., 1999. Palinurus elephas: langouste rouge. http://www.mnhn.fr/mnhn/bimm/protection/fr/Especes/Fiches/Palinuruselephas.html, 2000-07-31
O'Brien, C.E., Jozet-Alves, C., Mezrai, N., Bellanger, C., Darmaillacq, A.-S. & Dickel, L., 2017. Maternal and Embryonic Stress Influence Offspring Behavior in the Cuttlefish Sepia officinalis. Frontiers in Physiology 8, 981.
Palero, F., Abelló, P., Macpherson, E., Beaumont, M. & Pascual, M., 2011. Effect of oceanographic barriers and overfishing on the population genetic structure of the European spiny lobster (Palinurus elephas). Biological Journal of the Linnean Society, 104, 407-418
Palero, F., Abelló, P., Macpherson, E., Gristina, M. & Pascual, M., 2008. Phylogeography of the European spiny lobster (Palinurus elephas): influence of current oceanographical features and historical processes. Molecular Phylogenetics and Evolution, 48, 708-717.
Perry, S. L., 2015. Living seas: future fisheries; assessment of welsh fisheries. Report by The Wildlife Trust of south and west Wales. Living Seas Report No: 2, 109pp.
Phillips, B., 2013. Lobsters : Biology, Management, Aquaculture and Fisheries, Somerset, United Kingdom: John Wiley & Sons, Incorporated
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Quetglas, A., Gaamour, A., Reñones, O., Missaoui, H., Zarrouk, T., Elabed, A. & Goñi, R., 2004. Common spiny lobster (Palinurus elephas Fabricius 1787) fisheries in the western Mediterranean: A comparison of Spanish and Tunisian fisheries. 47, 63-80.
Rjeibi, O., Gaamour, A. & Missaoui, H., 2010. Kinetics of Oögenesis and Spawning Strategy of the Red Spiny Lobster Palinurus elephas. 30, 401-412.
Russell, F.S., 1927. The vertical distribution of marine macroplankton. V. The distribution of animals caught in the ring-trawl in daytime in the Plymouth area. Journal of the Marine Biological Association of the United Kingdom, 14, 557-607.
Russell, F.S., 1973. A summary of the observations on the occurrence of planktonic stages of fish off Plymouth 1924-1972. Journal of the Marine Biological Association of the United Kingdom, 53, 347-355.
Shields, J.D., 2011. Diseases of spiny lobsters: A review. Journal of Invertebrate Pathology, 106, 79-91
Slater, M., 2015. Cornwall Seasearch Surveys - Summary Report 2015. Seasearch Report. Truro: Cornwall Wildlife Trust.
Slater, M., 2017. Annual report 2017. Seasearch Report. Ross-on-Wye: The Marine Conservation Society.
Stanley, J.A., Radford, C.A., Jeffs, A.G., 2012. Location, location, location: finding a suitable home among the noise. Proceedings of the Royal Society B 279, 3622-3631.
Trindade-Gonçalves, D., 2010. Spiny lobster fishery on the southwest coast of Portugal: Conciliating fisheries and conservation (In Portuguese).MA thesis: Universidade do Algarve.
Tully, O., Heffernan, O., Robinson, M., Lawler, I., O'Leary, A., McFadden, Y., Addison, J., Bell, M., Smith, M., Latrouite, D., Freire, J., Sobrino, I., Goni, R., Carbonell, A., Maunou, F., Sarda, F., Company, J., Silva, C., Queroga, H., Belcari, P., Sbrana, M., Sartor, P., Mytilineou, C., Politou, C.Y., Kavadas, S., Conides, A., Yafidis, D., Moksnes, P.O., Pickering, H., White, H., Jensen, A., Collins, K. & Smith, P., 2003. Biology of European Decapod Crustaceans. Workpackage 1 Report, European Decapod Fisheries: Assessment and Management (EDFAM), pp 56. Available from: http://imbriw.hcmr.gr/en/wp-content/uploads/2014/02/EDFAM.pdf
Vaquer-Sunyer, R. & Duarte, C.M., 2008. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences, 105, 15452.
Wänke, A., 2018. Ca2+, Mg2+ and urate: Hemocyanin modulators of the European spiny lobster (Palinurus elephas). Characterisation of their binding to hemocyanin and their effects on hemocyanin's oxygen binding. Ph.D Thesis, Heinrich Heine University Düsseldorf. Available from:https://docserv.uni-duesseldorf.de/servlets/DocumentServlet?id=10314
Waterman, T. (2012). Metabolism and Growth. Saint Louis: Elsevier Science.
Wilson, D.P., 1951. A biological difference between natural sea waters. Journal of the Marine Biological Association of the United Kingdom, 30, 1-19.
- Goñi, R., Hilborn, R., Díaz, D., Mallol, S. & Adlerstein, S., 2010. Net contribution of spillover from a marine reserve to fishery catches. Marine Ecology Progress Series, 400, 233-243.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Isle of Wight Local Records Centre, 2017. IOW Natural History & Archaeological Society Marine Invertebrate Records 1853- 2011. Occurrence dataset: https://doi.org/10.15468/d9amhg accessed via GBIF.org on 2018-09-27.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Nottinghamshire Biological and Geological Records Centre, 2018. Records of Crayfish in Nottinghamshire. Occurrence dataset: https://www.nottinghamcity.gov.uk/events-markets-parks-and-museums/parks-and-open-spaces/nottinghamshire-biological-and-geological-record-centre-nbgrc/ accessed via NBNAtlas.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-30
Citation
This review can be cited as:
Last Updated: 13/11/2020
- spiny lobster
- crawfish