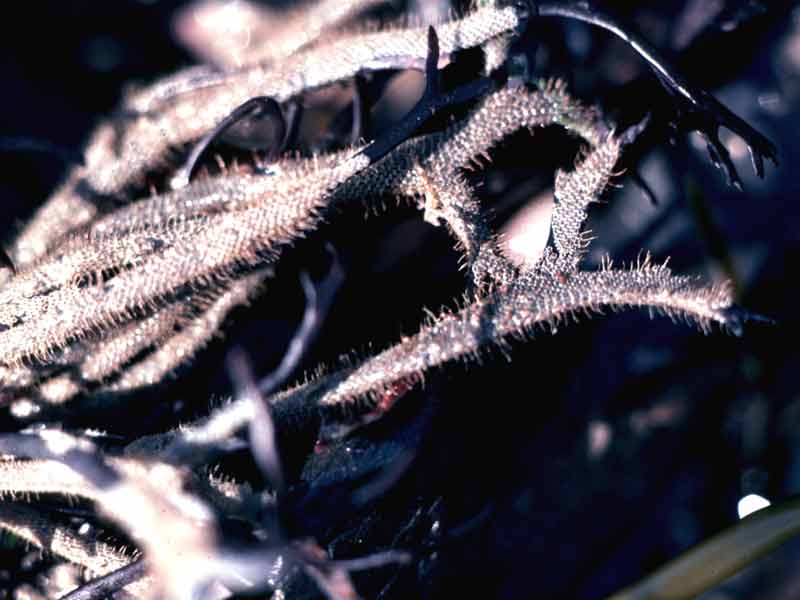
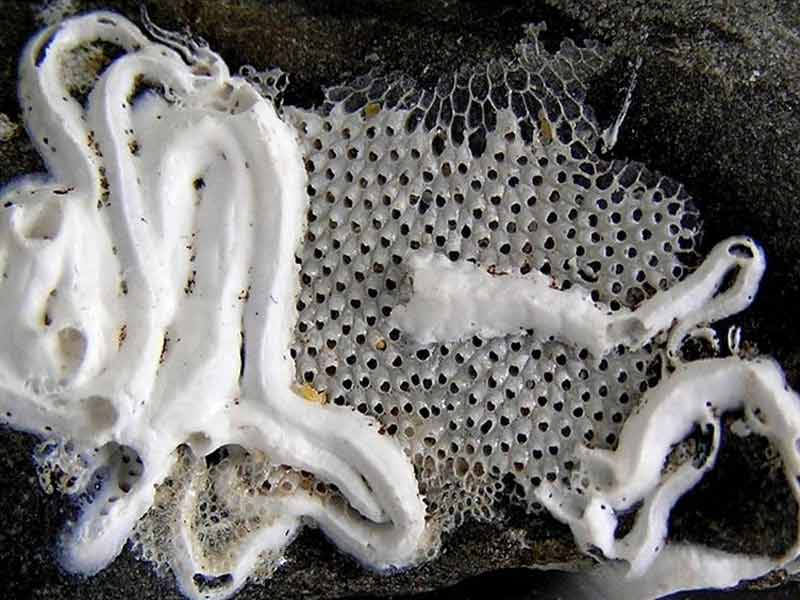
Thorny sea mat (Electra pilosa)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Harvey Tyler-Walters | Refereed by | Dr Peter J. Hayward |
| Authority | (Linnaeus, 1767) | ||
| Other common names | - | Synonyms | - |
Summary
Description
Electra pilosa may form star shaped or broad sheet colonies on the fronds of large algae (e.g. Laminaria and fucoids), small irregular patches on stones and shells, narrow tufts (independent of the substratum), or cylindrical incrustations around the fronds of small red algae (e.g. Mastocarpus stellatus). The zooids are ovate-oblong in shape, typically 0.5-0.6 by 0.25-0.35 mm. About half the front of the zooid is calcified but translucent, perforated by large pores, leaving an oval, membranous, frontal area distally, surrounded by 4-12 (often 9) spines. Spines vary in length but the median, proximal spine is always present and usually larger than the rest, although in some cases it may become well developed and longer than the zooid giving the colony a hairy appearance.
Recorded distribution in Britain and Ireland
Common on all coasts of the British Isles, although under recorded on parts of the east coast.Global distribution
Common in all temperate seas.Habitat
Colonizes a variety of substrata in marine habitats from low water into the shallow sublittoral, particularly macroalgae such as Fucus serratus and laminarians.Depth range
Intertidal to at least 50mIdentifying features
- Ooecia, avicularia and vibracula absent.
- About half the front of the zooid calcified (the gymnocyst) and perforated by large pores.
- Autozooids oval-oblong, 0.5-0.6 by 0.25-0.35 mm.
- Zooids arranged in a quincunx pattern, i.e. four zooids surrounding a central one, however, zooids may lie side by side in extended linear growth.
- Four to twelve but usually nine spines present.
- Median proximal spine prominent and often of great length.
- Operculum simple and transparent.
- Polypide with 11 to 15 tentacles.
- Larvae free swimming (cyphonautes), not brooded.
Additional information
Colonies of Electra pilosa growing on erect substrata (e.g. a hydroid) may continue to grow lengthways once they have used up the available substratum, forming narrow, bilaminar fronds of zooids side by side, once described as Electra verticillata. Colonies growing on small pieces of substratum (e.g. a shell) occasionally enclose the substratum forming an unattached spherical colony, 3-7cm in diameter (Hayward & Ryland, 1998).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Bryozoa | Sea mats, horn wrack & lace corals |
| Class | Gymnolaemata | Naked throat bryozoans |
| Order | Cheilostomatida | |
| Family | Electridae | |
| Genus | Electra | |
| Authority | (Linnaeus, 1767) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | |||
| Male size at maturity | |||
| Female size range | Small-medium (3-10 cm) | ||
| Female size at maturity | |||
| Growth form | Crustose hard | ||
| Growth rate | See additional information | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Active suspension feeder | ||
| Diet/food source | Planktotroph | ||
| Typically feeds on | Phytoplankton, algal spores. | ||
| Sociability | Colonial | ||
| Environmental position | Epifaunal | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | See additional information | ||
Biology information
Growth form. Electra pilosa displays a variety of growth forms, such as stellate patches on flat substrata, cylindrical growths around algae to narrow tufts, and narrow bilaminar fronds or occasionally as spherical masses around small substrata, described earlier (see Ryland, 1967, 1976; and Silén, 1987 for reviews). The median proximal spine may become greatly elongated in response to overgrowth by colonies of other bryozoans (Stebbing, 1973; Ryland, 1976) or in response to wave-related abrasion by algae (Bayer et al., 1997). The extended spine may protect the feeding polypide from physical or mechanical disturbance (Bayer et al., 1997).
Growth rates. Growth rates in bryozoans have been shown to vary with environmental conditions, especially water flow, food supply, temperature, competition for food and space, and genotype. For example:
- Best & Thorpe (1986) reported that feeding rate increased with increasing food concentration;
- Bayer et al. (1994) noted that variation in growth rates between colonies due to genotype was greater than that due to food ration;
- Okamura (1988) reported that in epiphytic communities, feeding rate increased with increasing flow (from 0.01-0.02 m/sec to 0.1-0.12m/s) but was reduced by competing bryozoan communities (Alcyonidium sp. or Flustrellidra hispida) in slow flow but enhanced by them in fast flow conditions; and
- bryozoans studied (inc. Electra pilosa) in natural currents in the Menai Strait, fed adequately and maintained growth even in very high current flows (Hermansen, et al., 2001).
- Although growth rates increased with temperature, zooid size decreased, which may be due to increased metabolic costs at higher temperatures (Menon, 1972; Ryland, 1976; Hunter & Hughes, 1994). In the Menai Straits, larger zooids are produced in spring at times of peak phytoplankton primary productivity but mean zooid size decreased as temperatures increased in summer (Okamura, 1987 cited in Hunter & Hughes, 1994).
Growth rates of 0.1-0.12 µm/day were reported, irrespective of flow regime, and genotype, while the natural population was reported to grow at ca 0.08 µm/ day (Hermansen et al., 2001).
Feeding. The structure and function of the bryozoan lophophore were reviewed by Ryland (1976), Winston, (1977) and Hayward & Ryland (1998). Best & Thorpe (1994) suggested that intertidal Bryozoa would probably be able to feed on small flagellates, bacteria, algal spores and small pieces of abraded macroalgae.
Allergenic response. Electra pilosa and other bryozoans have been reported to cause dermatitis and occupational eczema in fishermen (Ryland, 1967; Jeanmougin et al., 1987 summary only).
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Strait or Sound, Sea loch or Sea lough, Ria or Voe, Estuary, Enclosed coast or Embayment |
| Biological zone preferences | Lower eulittoral, Lower infralittoral, Mid eulittoral, Sublittoral fringe, Upper infralittoral |
| Substratum / habitat preferences | Macroalgae, Artificial (man-made), Bedrock, Caves, Cobbles, Large to very large boulders, Other species (see additional information), Overhangs, Small boulders, Under boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Moderately exposed, Sheltered |
| Salinity preferences | Full (30-40 psu) |
| Depth range | Intertidal to at least 50m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Electra pilosa may be found on most substrata, and is part of the epiphytic fauna of macroalgae such as Fucus serratus in the intertidal and the stipes or holdfasts of laminarians in the subtidal. Electra pilosa may also be found encrusting the shells of shellfish such as mussels. It is also a common member of the fouling community (Ryland, 1967). The abundance of bryozoans is positively correlated with supply of hard substrata and hence with current strength (Eggleston, 1972b; Ryland, 1976). Similarly, the abundance of Electra pilosa increased with increasing fucoid density and surface area (O'Connor et al., 1979).Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Permanent (synchronous) hermaphrodite |
| Reproductive frequency | Annual episodic |
| Fecundity (number of eggs) | |
| Generation time | <1 year |
| Age at maturity | Insufficient information |
| Season | August - September |
| Life span | Insufficient information |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | See additional information |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period |
Life history information
Reproduction. Bryozoan colonies are hermaphrodite, however, zooids may be monoecious, dioecious, protandrous or protogynous, depending on the species (Hayward & Ryland, 1998). Sperm are shed from pores in the polypide tentacles of male zooids (Hayward & Ryland, 1998). In Electra pilosa maternal lophophores may actively collect sperm (Temkin, 1996). The ovaries produce up to 31 oocytes of 121-145 µm in diameter, which are released into the coelomic cavity. Temkin (1996) has shown recently that fertilization is internal, rather than external as thought previously (see Reed, 1991). Eggs come into contact with sperm (either as aggregates or singly) in the coelomic cavity, fertilization occurring at or near ovulation (Temkin, 1996). Embryos are shed into the water column and develop into planktonic cyphonautes larvae (Ryland, 1976; Reed, 1991; Hayward & Ryland, 1998). Entrainment of released sperm by the tentacles of feeding polypides in bryozoans may reduce dispersal, resulting in self-fertilization (Temkin, 1996).
Fecundity. Individual zooids may produce up to 31 eggs and potentially the same number of embryos (with a fertilization efficiency of 83-100%) (Temkin, 1994), although Hyman (1959) reported a maximum of 17 eggs being released. However, while each individual zooid is not prolific, the fecundity of the colony is probably directly proportional to the number of functional zooids (Bayer et al., 1994).
Reproductive season. Colonies containing eggs and sperm are present in August and September and cyphonautes larvae are present in the plankton throughout the year (Hayward & Ryland, 1998). Electra pilosa was reported to settle between April and the end of November, with peaks in May/June and July to August (Ryland, 1967).
Longevity. Bayer et al. (1994) reported that colonies of Electra pilosa maintained in the laboratory died from the inside out, i.e. after several months the central part of the colony generally began to die. They noted that longevity data for Electra pilosa colonies was not available. However, although Electra pilosa colonies could probably survive for several years, it is probably adapted to ephemeral habitats, capable of rapid growth and reproduction of numerous offspring (r-selected).
Recruitment. Bryozoan larvae are probably sensitive to surface contour, chemistry and the proximity of conspecific colonies. However, Hayward & Ryland (1998) suggested that larval behaviour at settlement is only of prime importance to species occupying ephemeral habitats. For example, Electra pilosa larvae tend to orientate themselves with water flow along Fucus serratus fronds (the trend increasing with wave action) (Ryland, 1977), prefer to settle at the distal ends of the fronds and on the concave surfaces of the seaweed (Seed, 1985). (Eggleston, 1972b) demonstrated that the number and abundance of species of bryozoan increased with increased current strength, primarily due to a resultant increase in the availability of stable, hard substrata (Eggleston, 1972b; Ryland, 1976). Ryland (1976) reported that significant settlement in bryozoans was only found near a reservoir of breeding colonies. Ryland (1977) suggested that marine bryozoan larvae tend to settle on the underside of submerged structures or in shaded habitats, possibly due to avoidance of accumulated sediment or competition from algae. However, Electra pilosa larvae have an extended planktonic life and this species is a common member of fouling communities, and occurs on buoys where many other species of bryozoa are unable to colonize (Ryland, 1967). Therefore, Electra pilosa probably exhibits good dispersal and potentially very rapid recruitment.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceRemoval of the substratum, be it shell, rock, or macroalgae will result in removal of the attached colonies of Electra pilosa. Therefore, an intolerance of high has been recorded. Recoverability is likely to be very high (see additional information below). | High | Very high | Low | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering by 5 cm of sediment is likely to prevent feeding, and hence growth and reproduction, as well as respiration. In addition, associated sediment abrasion may remove the bryozoan colonies or the macroalgae on which they grow. A layer of sediment will probably also interfere with larval settlement. Therefore, an intolerance of high has been recorded. Recoverability has been assessed as very high (see additional information below). | High | Very high | Low | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceThe abundance of most bryozoan species declines with increasing suspended sediment loads. However, Moore (1973c; 1977) regarded Electra pilosa to be ubiquitous with respect to turbidity in subtidal kelp holdfasts in north east England. Seed (1985) also suggested that Electra pilosa was relatively tolerant of silt-laden habitats, although O'Connor et al. (1979) noted that the abundance of Electra pilosa in epiphytic communities on Fucus serratus in Strangford Lough, northern Ireland, was reduced slightly in response to increasing suspended sediment levels. Its abundance tended to increase at the distal ends of fronds exposed to increased sediment loads, presumably since the distal portions of fronds were held further above the substratum when immersed. However, the abundance of the competing bryozoan Flustrellidra hispida decreased markedly with increased sediment load. Overall, Electra pilosa is a relatively silt tolerant bryozoan and an intolerance of low has been recorded at the benchmark level. Recoverability is probably immediate. However, in highly turbid estuaries Electra pilosa may be excluded. In addition, epiphytic communities may be more intolerant since the macroalgae on which they live are likely to be excluded by high suspended sediment loads due to the increased turbidity. | Low | Immediate | Not sensitive | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceA decrease in suspended sediment may reduce the availability of organic particulates. However, a decrease in particulates is likely to encourage the settlement and growth of bryozoans including Electra pilosa. Therefore, tolerant* has been recorded. A decrease in sediment load is also likely to allow competitors such as other bryozoans and ascidians to colonize the habitat. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Desiccation [Show more]Desiccation
EvidenceColonies epiphytic on macroalgae are probably protected from desiccation by the humid environment provided by the algal fronds. However, Wood & Seed (1980) noted a marked decrease in abundance of Electra pilosa on Fucus serratus with increasing shore height. Therefore, an increase in desiccation is likely to reduce the extent and abundance of the population and an intolerance of intermediate has been recorded. Recoverability is likely to be very high. Subtidal populations are unlikely to experience desiccation. | Intermediate | Very high | Low | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceIncreased emergence will expose populations to increased risk of desiccation (above), increased extremes of temperature, and decrease the length of time available for feeding. Wood & Seed (1980) noted a marked decrease in abundance of Electra pilosa on Fucus serratus with increasing shore height, although colonies were reported on fucoids exposed for 5hrs on spring tides or ca 6hrs on neap tides in their shaded study site (reduced desiccation). Therefore, an increase in emergence is likely to result in a decrease in the abundance and extent of intertidal populations of Electra pilosa and an intolerance of intermediate has been reported. Recoverability is likely to be rapid. Subtidal populations are unlikely to be exposed to this factor. | Intermediate | Very high | Low | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceA decrease in emersion will decrease the risk of desiccation and effectively provide additional habitat for colonization, potentially allowing the Electra pilosa population, and its macroalgal substrata, to increase in extent. Therefore tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceWater flow has been shown to be important for the development of bryozoan communities and the provision of suitable hard substrata for colonization (Eggleston, 1972b; Ryland, 1976). In addition, areas subject to high mass transport of water such as the Menai Strait, Norwegian fjords or tidal rapids generally support large numbers of bryozoan species. Although, active suspension feeders, their feeding currents are probably fairly localized and they are dependant on water flow to bring adequate food supplies within reach. Hermansen et al. (2001) grew colonies of Electra pilosa colonies on rafts exposed to currents of 0.01-0.15m/sec. O'Connor et al. (1979) reported that the abundance of Electra pilosa on Fucus serratus declined with increasing current speed (up to 0.5m/sec), however, the observed trend may be due to an increase in abundance of competing bryozoans as current speed increased. Ryland (1970) noted that in turbulent regions of tidal rapids in Lough Ine, Ireland, the bryozoan species present were typical of wave exposed coasts and included Electra pilosa. An increase of water flow from weak to strong will probably have little adverse effects, however if water flow increased to very strong, feeding efficiency, and hence growth, would probably be reduced. Therefore, an intolerance of low has been reported. Recoverability has been assessed as immediate. | Low | Immediate | Not sensitive | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceA decrease in water flow is likely to increase siltation and potentially decrease food availability. Increased siltation may result in increased smothering of surfaces, and a reduction in settlement by bryozoan larvae (see above). Okamura (1988) reported that water flow stimulated feeding in Electra pilosa, so that feeding rates were reduced in slow water flow. Although, Electra pilosa is tolerant of silt-laden conditions, it is still probably dependant on water flow to bring an adequate supply of food within range of their feeding currents. Therefore, a reduction in water flow from moderately strong to very weak will probably result in a reduction in the abundance of Electra pilosa colonies and an intolerance of intermediate has been recorded. Recoverability has been assessed as very high. | Intermediate | Very high | Low | Low |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceGrowth rates were reported to increase with temperature, however, zooid size decreased, which may be due to increased metabolic costs at higher temperature (Menon, 1972; Ryland, 1976; Hunter & Hughes, 1994). The final size of the colony after prolonged exposure also declines at higher temperate (Menon, 1972). In the Menai Straits larger zooids are produced in spring at times of peak phytoplankton primary productivity but mean zooid size decreased as temperatures increased in summer (Okamura, 1987 cited in Hunter & Hughes, 1994).Menon (1972) reported that the upper lethal temperature and median lethal temperature varied significantly with acclimation temperature but found no obvious correlation with season, e.g. 24hr upper lethal temperature was ca 25 °C in colonies acclimated to 5 °C but ca 29 °C when acclimated to 22 °C (Menon, 1972). Electra pilosa is widely distributed in temperate seas both north and south of the British Isles and is probably tolerant to chronic long term change in temperature in British waters. However, acute temperature change may affect growth, feeding and hence reproduction, and an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Low |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceIntertidal populations may be exposed to low temperatures and frost but would probably receive some protection amongst the fronds of their macroalgal host. Electra pilosa is widely distributed in temperate seas, occurring as far north as the Barents Sea within the Arctic Circle (Gontar & Denisenko, 1989). Menon (1972) reported that individual zooids on the growing rim of colonies survived when kept at -4 °C for 14 days, although the inner zooids died. Menon (1972) demonstrated that all the zooids on the rim of colonies acclimated to 6 °C for 6 months before being kept in ice at -4 °C for 14 days, although apparently killed regenerated when returned to 6 °C. Therefore, Electra pilosa is unlikely to be adversely affected by long term temperature change in British waters. Hyman (1959) reported that a reduction in temperature of only 3 °C was enough to interrupt feeding, and that Electra pilosa colonies became un-responsive at 4 °C. Therefore, acute short term decreases in temperature may interfere with feeding and hence reproduction and an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Moderate |
Increase in turbidity [Show more]Increase in turbidity
EvidenceAn increase in turbidity is likely to result in a decrease in phytoplankton and macroalgal primary production, which may reduce food available to Electra pilosa. Therefore, an intolerance of low has been recorded. | Low | Immediate | Not sensitive | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceAn decrease in turbidity may increase primary productivity and food available for Electra pilosa. However, it is unlikely to be adversely affected, so tolerant has been recorded. | Tolerant | Not relevant | Not sensitive | Moderate |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceElectra pilosa is probably tolerant of wave exposure and is probably capable of occupying niches, overhangs and underboulder habits on even the most exposed shores. However, it reaches its highest abundance on macroalgal hosts within the intertidal, e.g. Fucus serratus which are likely to be lost from wave exposed shores. Populations on the holdfasts of laminarians will probably be unaffected by increases of wave action, until very or extremely exposed conditions, at which point the abundance of kelps would decline. Therefore, an intolerance of intermediate has been recorded to represent reduced population abundance with increasing wave action. Recoverability has been assessed as very high (see additional information below). Increased wave action will also increase the degree of abrasion from algae or sediment (see below). | Intermediate | Very high | Low | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceA decrease in wave exposure from, for example, moderately exposed to very sheltered is likely to increase the availability of macroalgae for colonization by Electra pilosa. However, increased shelter may also increase siltation (see above). Decreased wave action is likely to reduce abrasion by algae or sediment (see below). Overall, in areas subject to tidal streams or currents a decrease in wave action may not adversely affect the population. However, in areas where the main source of water flow over the substratum (rock or macroalgae) is caused by wave action a reduction in wave exposure to for example very sheltered may be detrimental, due to reduced food availability. Therefore, an intolerance of low has been recorded. Recoverability is likely to be immediate. | Low | Immediate | Not sensitive | Low |
Noise [Show more]Noise
EvidenceThe species is unlikely to be sensitive to changes in noise vibrations. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceThe species is unlikely to be sensitive to changes in visual perception. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceBayer et al. (1997) demonstrated that abrasion by seaweeds, rather than wave crash itself, induced the formation of extended median proximal spines in Electra pilosa, presumably to protect the lophophore from physical damage. As a major epiphyte of macroalgae, Electra pilosa is probably tolerant of seaweed abrasion. However, abrasion or physical disturbance by an anchor would probably destroy the colonies. In addition, physical disturbance by an anchor or passing scallop dredge is likely to remove a proportion of large macroalgae, such as fucoids and laminarians. However, even though the colonies eventually die as the substratum rots, over a few weeks at sea they are likely to shed thousands of larvae, and seaweed rafts are now seen as important dispersal agents (P. Hayward, pers. comm.). Overall, a low intolerance has been suggested. Recoverability is likely to be very high (see additional information below). | Low | Very high | Very Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceRemoval of a colony from its substratum would probably be fatal, and encrusting bryozoa are not known to be able to reattach. Therefore, an intolerance of high has been recorded. Recoverability is likely to be very high. | High | Very high | Low | High |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceBryozoans are common members of the fouling community, and amongst those organisms most resistant to antifouling measures, such as copper-containing anti-fouling paints (Soule & Soule, 1977; Holt et al., 1995). Bryan & Gibbs (1991) reported that there was little evidence regarding TBT toxicity in bryozoa with the exception of the encrusting Schizoporella errata, which suffered 50% mortality when exposed for 63 days to 100ng/l TBT. Rees et al. (2001) reported that the abundance of epifauna (including bryozoans) had increased in the Crouch estuary in the five years since TBT was banned from use on small vessels. This last report suggests that bryozoans may be at least inhibited by the presence of TBT. Hoare & Hiscock (1974) suggested that polyzoa were amongst the most sensitive species to acidified halogenated effluents in Amlwch Bay, Anglesey. Electra pilosa occurred at low abundance on laminarian holdfasts within the bay, compared to sites outside the affected area. Therefore, an intolerance of intermediate has been recorded. Recoverability is probably very high (see additional information below). | Intermediate | Very high | Low | Low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryozoans are common members of the fouling community, and amongst those organisms most resistant to antifouling measures, such as copper-containing anti-fouling paints (Soule & Soule, 1977; Holt et al., 1995). Bryozoans were shown to bioaccumulate heavy metals to a certain extent (Holt et al., 1995). For example Bowerbankia gracilis and Nolella pusilla accumulated Cd, exhibiting sublethal effects (reduced sexual reproduction and inhibited resting spore formation) between 10-100 µg Cd /l and fatality above 500 µg Cd/l (Kayser, 1990). However, given the tolerance of bryozoans to copper based anti-fouling treatments, and assuming similar physiology between species, an intolerance of low has been recorded albeit with very low confidence. | Low | Immediate | Not sensitive | Low |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceLittle information on the effects of hydrocarbons on bryozoans was found. Ryland & de Putron (1998) did not detect adverse effects of oil contamination on the bryozoan Alcyonidium spp. or other sessile fauna in Milford Haven or St. Catherine's Island, south Pembrokeshire. Houghton et al. (1996) reported a reduction in the abundance of intertidal encrusting bryozoa (no species given) at oiled sites after the Exxon Valdez oil spill. Soule & Soule (1979) reported that the encrusting bryozoan Membranipora villosa was not found in the impacted area for 7 months after the December 1976 Bunker C oil spill in Los Angeles Harbour. Of the eight species of bryozoan recorded on the nearby breakwater two weeks after the incident, only three were present in April and by June all had been replaced by dense growths of the erect bryozoan Scrupocellaria diegensis. Mohammad (1974) reported that Bugula spp. and Membranipora spp. were excluded from settlement panels near a Kuwait Oil terminal subject to minor but frequent oil spills. Encrusting bryozoans are also probably intolerant of the smothering effects of oil pollution, resulting in suffocation of colonies. Therefore, given the above evidence of intolerance in other Membraniporidae, a intolerance of high has been recorded, albeit at low confidence. Recoverability is probably very high (see additional information below). | Intermediate | Very high | Low | Very low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficientinformation | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceA moderate increase in nutrient levels may increase the food available to Electra pilosa, either in the form of phytoplankton or detritus. However, no effects of nutrient enrichment on bryozoans were found. | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceRyland (1970) stated that, with a few exceptions, the Gymnolaemata were fairly stenohaline and restricted to full salinity (ca 35 psu). Subtidal populations are unlikely to be exposed to hypersaline effluents or conditions within British waters. Intertidal populations may be exposed to increased salinities due to evaporation during emersion. However, Electra pilosa is found predominately on the lower shore and is unlikely to encounter an increase in salinity at the benchmark level. | Not relevant | Not relevant | Not relevant | Not relevant |
Decrease in salinity [Show more]Decrease in salinity
EvidenceRyland (1970) stated that, with a few exceptions, the Gymnolaemata were fairly stenohaline and restricted to full salinity (ca 35 psu) and noted that reduced salinities result in an impoverished bryozoan fauna. However, Hyman (1959) reported that Membranipora membranacea, Electra pilosa and Acanthodesia tenuis were found in the upper parts of Chesapeake Bay, in variable salinities. In addition, Hyman (1959) reported that Electra pilosa retracted its lophophores when exposed to 31psu, adjusted to 20psu but died after 'some sojourn' at 17.5psu. Intertidal populations may be exposed to freshwater runoff or rainfall, and may be expected to demonstrate some level of tolerance. Therefore, Electra pilosa may survive a long term decrease in salinity from full to variable but colonies would probably die if exposed to reduced or low salinities, even in the short term. Therefore, a salinity of intermediate has been recorded at the benchmark level. Recoverability is probably very high (see additional information below). | Intermediate | Very high | Low | Moderate |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceNo information on the tolerance of Electra pilosa to changes in oxygenation was found. | No information | Not relevant | No information | Not relevant |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceNo information found. | No information | Not relevant | No information | Not relevant |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceElectra pilosa is unlikely to be subject to specific extraction. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceElectra pilosa is a common epiphyte on fucoids, especially Fucus serratus and laminarian holdfasts, e.g. Laminaria hyperborea, which are subject to extraction (see individual species reviews for details). Therefore, a proportion of the Electra pilosa population is likely to be removed with their host algae, and an intolerance of intermediate has been recorded. Recoverability is likely to be very high (see additional information below). | Intermediate | Very high | Low | Moderate |
Additional information
RecoverabilityElectra pilosa has a planktonic larvae with a protracted life in the plankton, potentially extended dispersal, and larvae settle between April and November (with peaks in May-June and July -October). Electra pilosa can also colonize a wide variety of substrata and is a common member of fouling communities. Therefore, it is likely to be able to colonize new habitats or free space rapidly, probably in 6 months or less. It grows and probably matures quickly, within a year or less, and subsequent expansion of the population and recovery of abundance, aided by the proximity of breeding colonies is also likely to occur rapidly, possibly within a few years at most.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Electra pilosa is the preferred prey for several species of sea slugs, Limacia clavigera, Polycera quadrilineata, Onchidoris muricata and Adalaria proxima. Adalaria proxima is almost entirely dependant on Electra pilosa and its veligers will only metamorphose in the presence of Electra pilosa (Ryland, 1976; Picton & Morrow, 1994). Bryozoans, including Electra pilosa are also preyed on by pycnogonids (sea spiders) such as Pycnogonum littorale or Achelia spp. and sea urchins such as Echinus esculentus and Psammechinus miliaris (Ryland, 1976). Moore (1973c) noted that the hairy form of Electra pilosa on kelp holdfasts in north east England acted as a substratum for the settlement of mussel spat.Bibliography
Bayer, M.M., Cormack, R.M. & Todd, C.D., 1994. Influence of food concentration on polypide regression in the marine bryozoan Electra pilosa (L.) (Bryozoa: Cheilostomata). Journal of Experimental Marine Biology and Ecology, 178, 35-50.
Bayer, M.M., Todd, C.D., Hoyle, J.E. & Wilson, J.F.B., 1997. Wave-related abrasion induces formation of extended spines in a marine bryozoan. Proceedings of the Royal Society of London, Series B, 264, 1605-1611.
Best, M.A. & Thorpe, J.P., 1986. Effects of food particle concentration on feeding current velocity in six species of marine Bryozoa. Marine Biology, 93, 255-262.
Best, M.A. & Thorpe, J.P., 1994. An analysis of potential food sources available to intertidal bryozoans in Britain. In Proceedings of the 9th International Bryozoology conference, Swansea, 1992. Biology and Palaeobiology of Bryozoans (ed. P.J. Hayward, J.S. Ryland & P.D. Taylor), pp. 1-7. Fredensborg: Olsen & Olsen.
Bruce, J.R., Colman, J.S. & Jones, N.S., 1963. Marine fauna of the Isle of Man. Liverpool: Liverpool University Press.
Bryan, G.W. & Gibbs, P.E., 1991. Impact of low concentrations of tributyltin (TBT) on marine organisms: a review. In: Metal ecotoxicology: concepts and applications (ed. M.C. Newman & A.W. McIntosh), pp. 323-361. Boston: Lewis Publishers Inc.
Eggleston, D., 1972b. Factors influencing the distribution of sub-littoral ectoprocts off the south of the Isle of Man (Irish Sea). Journal of Natural History, 6, 247-260.
Gontar, V.I. & Denisenko, N.V., 1989. Arctic Ocean Bryozoa. In The Arctic Seas. Climatology, oceanography, geology, and biology (ed. Y. Herman), pp.341-371. New York: Van Nostrand Reinhold Co.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hayward, P.J. & Ryland, J.S. 1998. Cheilostomatous Bryozoa. Part 1. Aeteoidea - Cribrilinoidea. Shrewsbury: Field Studies Council. [Synopses of the British Fauna, no. 10. (2nd edition)]
Hermansen, P., Larsen, P.S. & Riisgård, H.U., 2001. Colony growth rate of encrusting bryozoans (Electra pilosa and Celleporella hyalina). Journal of Experimental Marine Biology and Ecology, 263, 1-23.
Hoare, R. & Hiscock, K., 1974. An ecological survey of the rocky coast adjacent to the effluent of a bromine extraction plant. Estuarine and Coastal Marine Science, 2 (4), 329-348.
Holt, T.J., Jones, D.R., Hawkins, S.J. & Hartnoll, R.G., 1995. The sensitivity of marine communities to man induced change - a scoping report. Countryside Council for Wales, Bangor, Contract Science Report, no. 65.
Houghton, J.P., Lees, D.C., Driskell, W.B., Lindstrom & Mearns, A.J., 1996. Recovery of Prince William Sound intertidal epibiota from Exxon Valdez oiling and shoreline treatments, 1989 through 1992. In Proceedings of the Exxon Valdez Oil Spill Symposium. American Fisheries Society Symposium, no. 18, Anchorage, Alaska, USA, 2-5 February 1993, (ed. S.D. Rice, R.B. Spies, D.A., Wolfe & B.A. Wright), pp.379-411.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Hunter, E. & Hughes, R.N., 1994. Influence of temperature, food ration and genotype on zooid size in Celleporella hyalina (L.). In Proceedings of the 9th International Bryozoology Conference, Swansea, 1992. Biology and Palaeobiology of Bryozoans (ed. P.J. Hayward, J.S. Ryland & P.D. Taylor), pp. 83-86. Fredensborg: Olsen & Olsen.
Hyman, L.V., 1959. The Invertebrates, vol. V. Smaller coelomate groups. New York: McGraw-Hill.
Jeanmougin, M., Lemarchand-Venencie, F., Hoang, X.D., D'Hondt, J.L. & Civatte, J., 1987. Occupational eczema with photosensitivity due to contact with Bryozoa. Annales de Dermatologie et de Venereologie, 114, 353-358.
Kayser, H., 1990. Bioaccumulation and transfer of cadmium in marine diatoms, Bryozoa, and Kamptozoa. In Oceanic processes in marine pollution, vol. 6. Physical and chemical processes: transport and transformation (ed. D.J. Baumgartner & I.W. Duedall), pp. 99-106. Florida: R.E. Krieger Publishing Co.
Menon, N.R., 1972. Heat tolerance, growth and regeneration in three North Sea bryozoans exposed to different constant temperatures. Marine Biology, 15, 1-11.
Mohammad, M-B.M., 1974. Effect of chronic oil pollution on a polychaete. Marine Pollution Bulletin, 5, 21-24.
Moore, P.G., 1973c. Bryozoa as a community component on the northeast coast of Britain. In Living and fossil Bryozoa. Recent advances in research (ed. G.P. Larwood), pp. 21-36.
Moore, P.G., 1977a. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanography and Marine Biology: An Annual Review, 15, 225-363.
Nielsen, C., 1990. Bryozoa Ectoprocta. In Reproductive biology of invertebrates, vol. IV, part B. Fertilization, development, and parental care, (ed. K.G. Adiyodi & R.G. Adiyodi), pp. 185-200. Chichester: John Wiley & Sons.
O'Connor, R.J., Seed, R. & Boaden, P.J.S., 1979. Effects of environment and plant characteristics on the distribution of Bryozoa in a Fucus serratus L. community. Journal of Experimental Marine Biology and Ecology, 38, 151-178.
Okamura, B., 1988. The influence of neighbors on the feeding of an epifaunal bryozoan. Journal of Experimental Marine Biology and Ecology, 120, 105-123.
Picton, B. E. & Morrow, C.C., 1994. A Field Guide to the Nudibranchs of the British Isles. London: Immel Publishing Ltd.
Reed, C.G., 1991. Bryozoa. In Reproduction of marine invertebrates, vol. VI. Echinoderms and Lophophorates (ed. A.C. Geise, J.S. Pearse & V.B. Pearse), pp. 85-245. California: Boxwood Press.
Ryland, J.S., 1967. Polyzoa. Oceanography and Marine Biology: an Annual Review, 5, 343-369.
Ryland, J.S., 1970. Bryozoans. London: Hutchinson University Library.
Ryland, J.S., 1976. Physiology and ecology of marine bryozoans. Advances in Marine Biology, 14, 285-443.
Ryland, J.S., 1977. Taxes and tropisms of Bryozoans. In Biology of bryozoans (ed. R.M. Woollacott & R.L. Zimmer), pp. 411-436.
Seed, R., 1985. Ecological pattern in the epifaunal communities of coastal macroalgae. In The Ecology of Rocky Coasts: essays presented to J.R. Lewis, D.Sc. (ed. P.G. Moore & R. Seed), pp. 22-35. London: Hodder & Stoughton Ltd.
Silén, L., 1987. Colony growth pattern in Electra pilosa (Linnaeus) and comparable encrusting Cheilostome Bryozoans. Acta Zoologica (Stockholm), 68, 17-34.
Soule, D.F. & Soule, J.D., 1979. Bryozoa (Ectoprocta). In Hart, C.W. & Fuller, S.L.H. (eds), Pollution ecology of estuarine invertebrates. New York: Academic Press, pp. 35-76.
Stebbing, A.R.D., 1973. Observations on colony overgrowth and spatial competition. In Living and fossil Bryozoa (ed. G.P. Larwood), pp. 173-183. New York: Academic Press.
Winston, J.E., 1977. Feeding in marine bryozoans. In Biology of Bryozoans (ed. R.M. Woollacott & R.L. Zimmer), pp. 233-271.
Wood, V. & Seed, R., 1980. The effects of shore level on the epifaunal communities associated with Fucus serratus in the Menai Strait, North Wales. Cahiers de Biologie Marine, 21, 135-154.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Environmental Records Information Centre North East, 2018. ERIC NE Combined dataset to 2017. Occurrence dataset: http://www.ericnortheast.org.ukl accessed via NBNAtlas.org on 2018-09-38
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2014. Occurrence dataset: https://doi.org/10.15468/erweal accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2015. Occurrence dataset: https://doi.org/10.15468/xtrbvy accessed via GBIF.org on 2018-09-27.
Fife Nature Records Centre, 2018. St Andrews BioBlitz 2016. Occurrence dataset: https://doi.org/10.15468/146yiz accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Manx Biological Recording Partnership, 2017. Isle of Man wildlife records from 01/01/2000 to 13/02/2017. Occurrence dataset: https://doi.org/10.15468/mopwow accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2018. Isle of Man historical wildlife records 1995 to 1999. Occurrence dataset: https://doi.org/10.15468/lo2tge accessed via GBIF.org on 2018-10-01.
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
Merseyside BioBank., 2018. Merseyside BioBank (unverified). Occurrence dataset: https://doi.org/10.15468/iou2ld accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
Outer Hebrides Biological Recording, 2018. Invertebrates (except insects), Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/hpavud accessed via GBIF.org on 2018-10-01.
South East Wales Biodiversity Records Centre, 2023. SEWBReC Marine and other Aquatic Invertebrates (South East Wales). Occurrence dataset:https://doi.org/10.15468/zxy1n6 accessed via GBIF.org on 2024-09-27.
Suffolk Biodiversity Information Service., 2017. Suffolk Biodiversity Information Service (SBIS) Dataset. Occurrence dataset: https://doi.org/10.15468/ab4vwo accessed via GBIF.org on 2018-10-02.
Yorkshire Wildlife Trust, 2018. Yorkshire Wildlife Trust Shoresearch. Occurrence dataset: https://doi.org/10.15468/1nw3ch accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 13/08/2005