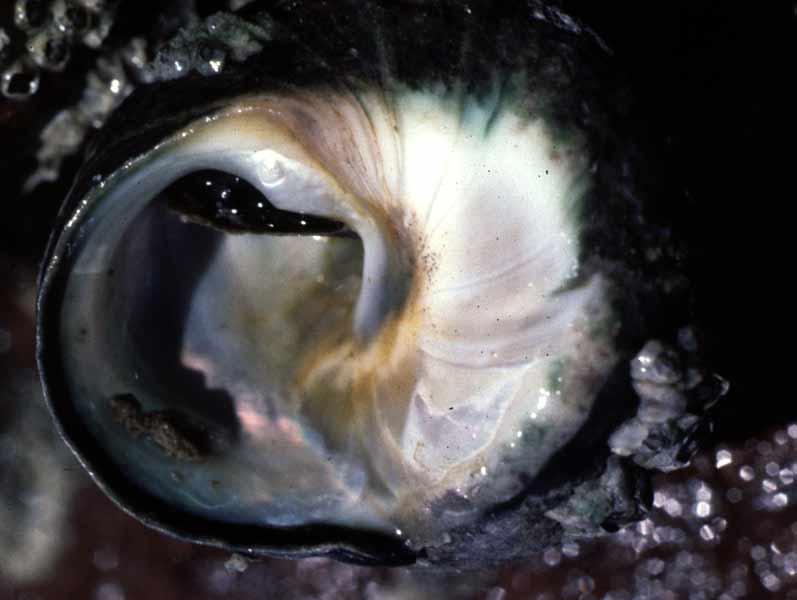
Thick top shell (Phorcus lineatus)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Nova Mieszkowska | Refereed by | This information is not refereed |
| Authority | (da Costa, 1778) | ||
| Other common names | Toothed top shell | Synonyms | Monodonta lineata (da Costa, 1778), Trochocochlea lineata , Turbo lineatus , Osilinus lineatus |
Summary
Description
Phorcus lineatus is the largest intertidal trochid in Britain. The shell is a turbinate spire consisting of up to six whorls. The last whorl dominates the shell and ends in a circular aperture, with a prominent 'tooth' or bulge on the nacreous (mother-of-pearl) inner shell layer. The outer lip of the aperture is thinner than the rest of the shell. Shell colour varies between dark green, grey and black, with complex brown or purple zigzag markings. The shell can reach up to 3cm in height (Graham, 1988) and 3cm basal diameter and is thicker and heavier than other species of topshell found in Britain. Shell growth lines are visible (Desai, 1966; Fretter & Graham, 1977; Williamson & Kendall, 1981). Erosion of the outer layer of the shell at the apex is common in older animals and the pearly inner layer of the shell can show through. The animal itself is mostly pink in colour. Two stalked eyes and a pair of sensory tentacles are located on the head above a well developed snout. The grey muscular foot lies underneath and slightly ventral to the head. Three pairs of sensory tentacles are present on the outside of the foot and are extended when the animal is active (Crothers, 2001).
Recorded distribution in Britain and Ireland
Abundant on rocky shores in Britain reaching its northern limits on Anglesey & eastern limits at Osmington Mills, Dorset (pers. obs.). Absent from Scotland & the east coast of England. Range from Churchtown to Malin Head in Ireland.
Global distribution
Found in the north eastern Atlantic from Morocco to Cap de la Hague, France on mainland Europe (Crisp & Southward, 1958; Fretter & Graham 1977). Northern limits reached in North Wales and North Ireland.
Habitat
Occurs in the midshore region of moderately exposed rocky shores in England and Wales. Requires a stable boulder field or broken shore with available bare rock.
Depth range
MHWS to MLWSIdentifying features
- Pronounced tooth or notch on aperture.
- Shell has up to 6 whorls.
- Shell dark green, grey or black with brown or red zigzag markings
- Underside pearly-white.
Additional information
Also commonly known as the toothed top shell. Irregular damage lines may be visible on the shell if the animal has experienced environmental shock or has been attacked by predators.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Gastropoda | Snails, slugs & sea butterflies |
| Order | Trochida | |
| Family | Trochidae | |
| Genus | Phorcus | |
| Authority | (da Costa, 1778) | |
| Recent Synonyms | Monodonta lineata (da Costa, 1778)Trochocochlea lineata Turbo lineatus Osilinus lineatus | |
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | 13-30 mm | ||
| Male size at maturity | 13+ mm | ||
| Female size range | 13+ mm | ||
| Female size at maturity | |||
| Growth form | Turbinate | ||
| Growth rate | See additional information | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Creeper | ||
| Characteristic feeding method | Grazer (surface/substratum) | ||
| Diet/food source | Herbivore | ||
| Typically feeds on | Microalgae | ||
| Sociability | No information | ||
| Environmental position | Epilithic | ||
| Dependency | Independent. None | ||
| Supports | None | ||
| Is the species harmful? | No | ||
Biology information
Growth rate. Initially rapid, Phorcus lineatus can grow up to 7-8mm in diameter between spawning and December (Fretter & Graham, 1977; Kendall et al.,1987), although the average size of newly settled animals is around 3 mm (pers. obs.). Growth slows down and may stop in the first winter and every successive winter, before increasing in spring (Williams, 1965). One year post-settlement, juveniles can reach 11 to 15 mm (Fretter & Graham 1977, Fretter, 1988). Growth slows in adults when they become sexually mature but continues throughout the life of the animal.
Feeding. Phorcus lineatus feeds on microscopic algae, which it grazes from rock surfaces using a brush-like radula on the tongue. Feeding is assumed to occur at night or during high water (Crothers, 2001) as no observations of feeding during daylight or at low water have been published.
Respiration. Phorcus lineatus has a gill for respiration in water and a well vascularised mantle cavity which allows the animal to breathe in air (Crothers, 2001).
Sensory. Phorcus lineatus detects its environment by means of two stalk eyes and a pair of sensory tentacles on the head, and three pairs of sensory tentacles on the foot.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Enclosed coast or Embayment, Estuary, Open coast, Sea loch or Sea lough, Strait or Sound |
| Biological zone preferences | Lower eulittoral, Mid eulittoral, Upper eulittoral |
| Substratum / habitat preferences | Bedrock, Large to very large boulders, Rockpools, Under boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.) |
| Wave exposure preferences | Exposed, Moderately exposed |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | MHWS to MLWS |
| Other preferences | No text entered |
| Migration Pattern | Seasonal (reproduction) |
Habitat Information
- Phorcus lineatus is widely distributed on rocky shores between the 20°C summer isotherm off Africa and the 6.5°C winter isotherm off Anglesey. Northern limits are primarily set by reproductive failure (Lewis et al., 1982; Lewis 1986; Kendall, 1986) but may also be determined by hydrography or unsuitable habitat (Crisp & Knight-Jones, 1953)
- Juvenile Phorcus lineatus are found in nursery areas underneath boulders or in fissures. Adults crawl out of these damp areas onto the sides of boulders during warm, dry periods but tend to retreat to the lower surfaces of rocks when the weather is colder.
- In early summer Phorcus lineatus adults migrate up the shore to the high eulittoral prior to spawning. Once spawning has occurred the animals migrate back down shore to the mid to lower eulittoral zone to overwinter.
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Gonochoristic (dioecious) |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | 100-1,000 |
| Generation time | 2-5 years |
| Age at maturity | 2 years |
| Season | May - August |
| Life span | 11-20 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Lecithotrophic |
| Duration of larval stage | 2-10 days |
| Larval dispersal potential | No information |
| Larval settlement period | Insufficient information |
Life history information
Reproductive cycle. Sexes are separate but the two sexes cannot be differentiated between by external examination (Fretter & Graham, 1977; Hickman, 1992). Phorcus lineatus has five stages to its reproductive cycle (Ortonet al., 1956; Desai, 1966). The onset of gonad maturation has been correlated with rising sea temperatures.
- Stage I Late summer the gonad is inactive. Both male and female gonads are brown in colour and appear as loose, sac-like structures. Any oocytes present are smaller than 25 µm in diameter.
- Stage II. In October the ovaries and testis both take on a greenish hue. Oocytes of up to 50 micrometres are present in females and spermatogonia are present in males.
- Stage III. In early January, ovaries and testis are green, oocytes have grown to diameters greater than 50 µm. Spermatocytes and spermatids are present in males.
- Stage IV. In February to May, ovaries are deep green in pigment and contain a mixture of mature and immature oocytes. Testis become pink in colour and contain spermatozoa.
- Stage V. In May, ovaries are deep green and distended, oocytes are mostly mature. Testis are pink/cream and contain fully active spermatozoa.
Spawning. Adult Phorcus lineatusmigrate upshore to the high eulittoral zone in early summer prior to spawning. It is thought that this migration brings the animals into a region of higher temperature required for spawning. Desai (1966) found that adults that had migrated furthest up shore were the first to spawn, supporting this idea. Phorcus lineatus is a broadcast spawner (Underwood, 1972; Hickman, 1992). Males release clouds of white spermatozoa into the water column and females undergo repeated spasms, releasing a few eggs at a time from the mantle cavity into the water (Fretter & Graham 1977). Fertilization occurs externally.
The breeding season is shorter near to northern range limits, with a single spawning period. Towards the centre of the range, the breeding season is longer and multiple spawning events occur (Garwood & Kendall, 1985, Bode et al,1986).
Larval development. Eggs of diameters between 165-250 µm are released individually (Desai, 1966; Fretter & Graham, 1994). The external jelly coating swells in contact with water, making the egg initially buoyant. After 20 minutes the jelly coating disintegrates and the egg sinks. The eggs are lecithotrophic (contain yolk) and provide food for larval development until the larvae hatch as free-swimming veligers after 29-30 hours. Six days after fertilization the larva has grown to approximately 1mm in diameter and has fully developed its crawling ability (Desai 1966, Fretter & Graham 1977). Larvae settle on the shore in the low eulittoral zone under boulders and in cracks and crevices.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceOsilinus lineatus is epifaunal so loss of the substratum would result in the loss of the population, and an intolerance of high has been recorded. Osilinus lineatus cannot survive on or move over substrate consisting of fine, mobile sediment. Recolonization, recruitment and recovery are possible once the substrate is restored, or to neighbouring areas of suitable habitat as the adults are capable of migration and the planktonic stage of the lifecycle will facilitate limited dispersal from neighbouring populations (see additional information below). | High | Moderate | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceSmothering by 5 cm of sediment is highly likely to cause death. Respiration would be prevented and the microalgal food source would not grow under such conditions. Smothering of nursery areas under boulders would also prevent survival of juvenile recruits which depend on a coarse grained substrate to settle and over-winter on. Recruitment failure may result from a chronic deposition over a year or an acute episode coinciding with the peak juvenile settlement season in autumn. This is evident in Aberaeron, where regular recruitment occurred throughout the 1970s and 1980s (Kendal, 1987) but the population was severely reduced in 2002 due to heavy sedimentation of the habitat (Kendal et al. in submission). Recoverability is likely to be moderate (see additional information below). | High | Moderate | Moderate | High |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceDeposition of suspended sediment may cause siltation of nursery areas, removing required habitat including nooks and crevices and prevent juveniles from settling and surviving. Recruitment failure may result from a chronic deposition over a year or an acute episode coinciding with the peak juvenile settlement season in autumn (see smothering above). Overall, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Low |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceOsilinus lineatus is unlikely to be adversely affected by a decrease in suspended sediment (editors addition). | Tolerant | Not relevant | Not sensitive | Low |
Desiccation [Show more]Desiccation
EvidenceOsilinus lineatus can fully retract into its shell and seal the opening with an operculum to minimize desiccation. This species can be mobile, feeding and walking during periods of low tide when the animal is exposed to air if conditions are damp or it is disturbed. It is tolerant of extended periods (days) exposed to air, and positions itself on the tops of boulders during sunny days when the desiccation risk is higher. It migrates upshore in the spring prior to spawning and remains there until the end of summer. This is an active movement into an area of increased desiccation stress which suggests that this species has a high tolerance. Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | High |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAdults migrate upshore prior to spawning to a region where the emergence period is greater than lower down the shore. When specimens are returned to the laboratory they can survive for a week without submergence in seawater. The species can therefore tolerate a wide emergence regime. | Low | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details Evidence | No information | |||
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceWater flow rates may become high enough to prevent animals from maintaining contact with the rock surface when moving and feeding at high tide. Increased flow rates may also move the boulder substrate around more, causing dislodgement and abrasion of the animals. Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | High |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceOsilinus lineatus is found in habitats with a range of flow rates including sheltered bays where flow rates are reduced. If the flow rate is too low and sediment drops out of suspension the habitat may be unsuitable for this species due to smothering of adults and nursery areas of juveniles (see smothering above). Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | High |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceIncreasing air and sea temperatures allows Osilinus lineatus to colonize areas previously too cold. This is evident around British coasts as this species has increased its range during the current period of climate warming (Kendal et al., in submission). Osilinus lineatus has a distribution range from Morocco to North Wales, where it reaches its northern limits. The average temperature gradient is approximately 6 °C between these locations. Osilinus lineatus can also tolerate the wide range of air and sea temperatures it is exposed to in the intertidal zone. | Tolerant* | Not relevant | Not sensitive* | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceA decrease in temperature may prevent regular successful recruitment within a population and could lead to a localized extinction. A sudden, sharp decrease in temperatures during the winter of 1962/63 resulted in the loss of many populations in Britain (Crisp, 1964). Some populations close to the northern limits have still not recovered. Overall, an intolerance of high to acute temperature decrease has been recorded. Recoverability is likely to be moderate (see additional information below). | High | Moderate | Moderate | Moderate |
Increase in turbidity [Show more]Increase in turbidity
EvidenceIncreases in turbidity will probably not have much of a direct effect on this species. Reduced irradiance may decrease the productivity of the microalgal food source. | Low | High | Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceOsilinus lineatus is typically found on moderately exposed shores but also occurs in small numbers on some shores where wave exposure is high. Individuals may survive an increase in wave exposure by becoming more cryptic in their habitat selection. If wave action is too strong the animals may not be able to maintain their hold on the rock surface and may be washed off. Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Moderate |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceOsilinus lineatus is also found in sheltered bays where there is little wave action. If the water movement is too low and sediment drops out of suspension the habitat may be unsuitable for this species due to smothering of adults and nursery areas of juveniles (see smothering above). | Low | High | Low | Moderate |
Noise [Show more]Noise
EvidenceThere is no evidence that noise adversely affects this species. They move towards localized drilling noise made by researchers on the shore (pers. obs.). | Low | Immediate | Not sensitive | Low |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceAdults do respond to visual presence by fully retracting into the shell, both when submerged and emersed. They do not close the operculum but remain attached to the rock by their foot. However, they re-emerge after a few minutes. | Low | Immediate | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceAbrasion can cause shell damage. The outer, thinner, dark layer of the shell is often abraded in older animals to the extent that the mother-of-pearl inner layer is evident on the top two or three whorls. Abrasion marks are more common on animals living on more exposed shores. Overall, an intolerance of low has been recorded. | Low | Moderate | Low | Moderate |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceAnimals displaced from the rock surface quickly reattach themselves if not carried away in the water column, when they may be lost. Animals that are displaced will attach to nearby substrate providing that it is suitable. Therefore, an intolerance of intermediate has been recorded. Recoverability is likely to be high (see additional information below). | Intermediate | High | Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceAdult Osilinus lineatus were seen to decline further in Milford Haven after the Sea Empress oil spill in 1996 (Little, 1999). | Intermediate | High | Low | Moderate |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceInsufficient | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceIt is unlikely that changes in nutrient levels will have a large effect on this species. Increase in the nutrient load of the water may lead to an increase in the microalgal food source. It has been suggested that toxic algal blooms may adversely affect this species but no direct evidence of this has been found. | Low | High | Low | Low |
Increase in salinity [Show more]Increase in salinity
EvidenceThis species is found on open ocean coasts adjacent to oceanic waters and can tolerate salinities of around 33-35, which are typical of large water bodies. They also inhabit rockpools in the high mid shore zone. These pools are exposed for around 6 hours every day and evaporation can cause the salinity of the remaining water to become very high. Osilinus lineatus can tolerate such an increase in salinity and an intolerance of low has been recorded. | Low | High | Low | Moderate |
Decrease in salinity [Show more]Decrease in salinity
EvidenceThis species is found in large estuaries such as the Bristol Channel where salinities are less than at open coast locations. It is also found higher up the shore where small trickles of freshwater occur on the rock. | Low | High | Low | Moderate |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceOsilinus lineatus has the ability to breathe in air as well as water, due to a well vascularised mantle cavity used for respiration along with their gill (Crothers, 2001). It actively removes itself from the water when the tide is retreating and the oxygen content of residual water decreases. This behaviour suggests a high intolerance to low oxygen content in water which has resulted in a behavioural adaptation. | High | Moderate | Moderate | Moderate |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceLichomolgid copepods have been recorded inside Osilinus lineatus. However, no information concerning the effect of such infestation was found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceIntroduction of non-native topshells occupying a similar zone on the shore (e.g. Gibbula pennanti) may increase competition for food resources. Depending on which species is the competitive dominant, Osilinus lineatus may reduce the width of its zone or maintain its distribution and force the invasive species further down the shore into the low shore zone. Therefore, an intolerance of intermediate has been recorded. Recoverability would be dependent on the removal of the non-native species, which is probably unlikely to occur. | Intermediate | Not relevant | NR | Low |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceIt is possible that Osilinus lineatus may be removed from the shore as misidentified Littorina littorea by winkle collectors due to their similar external appearance. On shores where Osilinus lineatus is rare or absent the topshell Steromphala umbilicalis can become more abundant in the mid shore zone where Osilinus lineatus would otherwise occur. This is likely to be the result of decreased competition for the detrital scraps of microalgae. | Intermediate | High | Low | Low |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceIt is possible that Osilinus lineatus may be removed from the shore as misidentified Littorina littorea by winkle collectors due to their similar external appearance. | Intermediate | High | Low | Low |
Additional information
Recoverability. Phorcus lineatus was severely affected on shores throughout Britain and Ireland during the cold winter of 1962/63 (Crisp (ed.), 1964a). Populations were completely wiped out at many sites in north and south Wales (Crisp, 1964b, Moyse & Nelson-Smith, 1964), and northeast Ireland (Boaden et al., 1964) and some have yet to become re-established. Recoverability has been low due to the latitudinal extent of the mortality, as this species has localized recruitment and there have been no neighbouring populations in the north of Wales to facilitate recolonization of these locations. It is highly likely that the range of Phorcus lineatus is temperature limited in Britain as range extensions past historical limits have occurred during the recent period of rapid climate warming (Kendal et al. in submission).
Phorcus lineatus is widely distributed on rocky shores between the 20°C summer isotherm off Africa and the 6.5°C winter isotherm off Anglesey. Northern limits are primarily set by reproductive failure (Lewis et al., 1982; Lewis, 1986; Kendall, 1987) but may also be determined by hydrography or unsuitable habitat (Crisp & Knight-Jones, 1953). Phorcus lineatus shows increased reproductive success under warmer conditions. Research suggests that the reproductive cycle is lengthened and multiple spawning events occur in locations with consistently higher temperatures of approximately 6°C than in Britain, typical of the coastal waters of Spain (Bode et al., 1986). If a population can reproduce successfully on a regular basis then it should be able to recover from detrimental physical alterations in the environment once the factor has been removed and conditions revert to their previous state. This is provided that enough mature adults survive or sufficient recruits from neighbouring populations settle in that location.
Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Phorcus lineatus may be taken as bycatch by people collecting the winkle Littorina littorea.Bibliography
Boaden, P.J.S., Gotto, R.V., Hartnoll, R.G. & Williams, G., 1964. North-east Ireland. Journal of Animal Ecology, 33, 197.
Bode, A., Lombas, I. & Anadon, N., 1986. Preliminary studies on the reproduction and population dynamics of Monodonta lineata and Gibbula umbilicalis (Mollusca, Gastropoda) on the central coast of Asturias (N. Spain). Hydrobiologia, 142, 31-39.
Crisp, D.J. & Knight-Jones, E.W., 1953. Discontinuities in the distribution of shore animals in North Wales. Report of the Bardsey Observatory, 29-34.
Crisp, D.J. & Southward, A.J., 1958. The distribution of intertidal organisms along the coasts of the English Channel. Journal of the Marine Biological Association of the United Kingdom, 37, 157-208.
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Crothers, J.H., 2001. Common topshells: an introduction to the biology of Osilinus lineatus with notes on other species in the genus. Field Studies, 10, 115-160.
Desai, B. N., 1966. The biology of Monodonta lineata (da Costa). Proceedings of the Malacological Society of London, 37, 1-17.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fretter, V. & Graham, A., 1977. The Prosobranch Molluscs of Britain and Ireland Part 2 –.Trochacea. Journal of Molluscan Studies, Supplement 3, 1-64.
Fretter, V. & Graham, A., 1994. British prosobranch molluscs: their functional anatomy and ecology, revised and updated edition. London: The Ray Society.
Garwood, P.R. & Kendall, M.A., 1985. The reproductive cycles of Monodonta lineata and Gibbula umbilicalis on the coast of mid-Wales. Journal of the Marine Biological Association of the United Kingdom, 65, 993-1008.
Graham, A., 1988. Molluscs: prosobranchs and pyramellid gastropods (2nd ed.). Leiden: E.J. Brill/Dr W. Backhuys. [Synopses of the British Fauna No. 2]
Hawkins, S. J. & Jones, H. D., 1992. Rocky Shores. London: Immel.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hickman, C.S., 1992. Reproduction and development of trochean gastropods. Veliger, 35, 245-272.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Kendall, M.A., 1987. The age and size structure of some northern populations of the trochid gastropod Monodonta lineata. Journal of Molluscan Studies, 53, 213-222.
Kendall, M.A., Williamson, P. & Garwood, P.R., 1987. Annual variation in recruitment and population structure of Monodonta lineata and Gibbula umbilicalis populations at Aberaeron, mid-Wales. Estuarine, Coastal and Shelf Science, 24, 499-511.
Lebour, M.V., 1937. The eggs and larvae of the British Prosobranchs with special reference to those living in the plankton. Journal of the Marine Biological Association of the United Kingdom, 22, 105-166.
Lewis, J.R., 1986. Latitudinal trends in reproduction, recruitment and population characteristics of some rocky littoral molluscs and cirripedes. Hydrobiologia, 142, 1-13.
Lewis, J.R., Bowman, R.S., Kendall, M.A. & Williamson, P., 1982. Some geographical components in population dynamics: possibilities and realities in some littoral species. Netherlands Journal of Sea Research, 16, 18-28.
Little, A., 1999. An autoecological study of Osilinus lineatus in the area affected by the Sea Empress oil spill. CCW Sea Empress Contract Report no. 322, 65 pp.
Moyse, J. & Nelson-Smith, A., 1964. Effects of the severe cold of 1962-63 upon shore animals in South Wales. Journal of Animal Ecology, 33, 183-190.
Orton, J.H., Southward, A.J. & Dodd, J.M., 1956. Studies on the biology of limpets II. The breeding of Patella vulgata L. in Britain. Journal of the Marine Biological Association of the United Kingdom, 35, 149-176.
Picton, B.E. & Costello, M.J., 1998. BioMar biotope viewer: a guide to marine habitats, fauna and flora of Britain and Ireland. [CD-ROM] Environmental Sciences Unit, Trinity College, Dublin.
Southward, A.J. & Crisp, D.J., 1954. The distribution of certain intertidal animals around the Irish coast. Proceedings of the Royal Irish Academy, 57B, 1-29.
Underwood, A.J., 1972. Observations on the reproductive cycles of Monodonta lineata, Gibbula umbilicalis and G. cineraria. Marine Biology, 17, 333-340.
Williams, E.E., 1965. The growth and distribution of Monodonta lineata (da Coasta) on a rocky shore in Wales. Field Studies, 2, 189-198.
Williamson, P. & Kendall, M.A., 1981. Population age structure and growth of the trochid Monodonta lineata determined from shell rings. Journal of the Marine Biological Association of the United Kingdom, 61, 1011-1026.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Cofnod – North Wales Environmental Information Service, 2018. Miscellaneous records held on the Cofnod database. Occurrence dataset: https://doi.org/10.15468/hcgqsi accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 17/04/2008