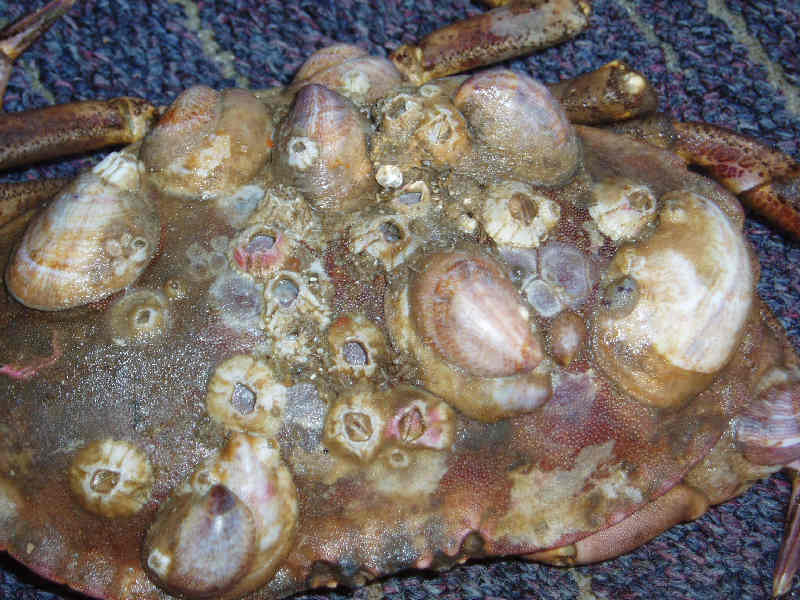
Slipper limpet (Crepidula fornicata)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Will Rayment | Refereed by | Dr Frédérique Viard |
| Authority | (Linnaeus, 1758) | ||
| Other common names | - | Synonyms | - |
Summary
Description
The shell is oval, up to 5 cm in length, with a much reduced spire. The large aperture has a shelf, or septum, extending half its length. The shell is smooth with irregular growth lines and white, cream, yellow or pinkish in colour with streaks or blotches of red or brown. Slipper limpets are commonly found in curved chains of up to 12 animals. Large shells are found at the bottom of the chain, with the shells becoming progressively smaller towards the top.
Recorded distribution in Britain and Ireland
In Britain, it is present on the east coast south of Spurn Head, the length of the south coast and northwards along the west coast to Cardigan Bay. It has been introduced accidentally to several locations in Ireland but a population has never persisted.
Global distribution
Originally found on the east coast of the Americas between Canada and Mexico. Now also introduced to British-Columbia, Washington state, Japan and Europe, where it is found on the Atlantic coast between Denmark and Spain, in Sicily and the Adriatic Sea.
Habitat
Crepidula fornicata is typically found attached to shells and stones on soft substrata around the low water mark and the shallow sublittoral. It is often attached to the shells of mussels Mytilus edulis and oysters Ostrea edulis.
Depth range
Low water mark to 60mIdentifying features
- Spire set posteriorly.
- Shell slightly curled dextrally.
- Shell smooth with irregular growth lines.
- Aperture elongate and oval.
- Septum extends from beneath spire for approximately half length of shell.
Additional information
Crepidula fornicata is a non-native species. The modern British population is known to have been introduced to Essex between 1887 and 1890 in association with oysters, Crassostrea virginica, imported from North America (Fretter & Graham, 1981; Eno et al., 1997).
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Mollusca | Snails, slugs, mussels, cockles, clams & squid |
| Class | Gastropoda | Snails, slugs & sea butterflies |
| Order | Littorinimorpha | |
| Family | Calyptraeidae | |
| Genus | Crepidula | |
| Authority | (Linnaeus, 1758) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | High density | ||
| Male size range | up to 5 cm | ||
| Male size at maturity | |||
| Female size range | Small-medium (3-10cm) | ||
| Female size at maturity | |||
| Growth form | Turbinate | ||
| Growth rate | 0.04-1.11mm/day | ||
| Body flexibility | None (less than 10 degrees) | ||
| Mobility | Sessile, permanent attachment | ||
| Characteristic feeding method | Active suspension feeder | ||
| Diet/food source | Detritivore, Planktotroph | ||
| Typically feeds on | Phytoplankton and particulate organic food | ||
| Sociability | Gregarious | ||
| Environmental position | Epibenthic | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No Edible | ||
Biology information
Abundance. In the Bay of Marennes-Oleron, France, Crepidula fornicata was found in a wide range of sediment grain sizes and depths. Maximum abundance and biomass reached 4770 individuals per m² and 354 g of dry weight per m² respectively in shallow muddy areas (De Montaudouin & Sauriau, 1999). Crepidula fornicata also occurs at "moderate" density, for example in the Arcachon Basin (De Montaudouin et al., 2001).
Size at maturity. Due to the protandrous hermaphroditic life-cycle of Crepidula fornicata, size at maturity is difficult to ascertain. Warne (1956), cited in Fretter & Graham (1981), reported size at maturity to be 4 mm but it is unclear whether this referred to both sexes or males only. Under laboratory conditions, Nelson et al. (1983) reported that the mean female length at first larval release was 23.8 mm.
Growth rate. Reported growth rates vary according to age. Pechenik et al. (1996) recorded juvenile growth rate for the nine days after metamorphosis as varying between 15 and 225 µm per day (mean 110.5 µm per day). Thouzeau(1991) recorded mean juvenile growth rates over one month following settlement as 38 to 48 µm per day with a maximum of 90 µm per day.
Mobility. Juvenile Crepidula fornicata are capable of slow crawling immediately after settlement and locating a suitable site for attachment and growth. This is either a stone or a chain of other Crepidula fornicata (conspecifics). The shell then grows to fit the substratum and consequently, most animals are incapable of further movement at the age of about two years (Fretter & Graham, 1981).
Nutrition. Following laboratory experiments, Thain (1984) deduced that, for optimum growth and reproduction, an individual Crepidula fornicata being fed with the alga Phaeodactylum tricornutum requires 5 x 108 algal cells per gram of flesh wet weight per day.
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Strait or Sound, Estuary, Enclosed coast or Embayment |
| Biological zone preferences | Lower circalittoral, Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Coarse clean sand, Cobbles, Fine clean sand, Gravel / shingle, Mixed, Mud, Muddy gravel, Muddy sand, Other species, Pebbles, Sandy mud, Small boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Very weak (negligible), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Sheltered, Very sheltered |
| Salinity preferences | Variable (18-40 psu) |
| Depth range | Low water mark to 60m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
Although Crepidula fornicata is a cosmopolitan species, which can tolerate a wide range of environmental conditions, populations are particularly well developed in wave protected areas such as bays, estuaries or sheltered sides of wave exposed islands (Blanchard, 1997). Similarly, the species is found on a variety of substrata but is most abundant in muddy or mixed muddy areas (De Montaudouin & Sauriau, 1999).
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | Protandrous hermaphrodite |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | 10,000-100,000 |
| Generation time | See additional information |
| Age at maturity | see additional information |
| Season | February - October |
| Life span | 5-10 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | Planktotrophic |
| Duration of larval stage | 1-2 months |
| Larval dispersal potential | Greater than 10 km |
| Larval settlement period | Insufficient information |
Life history information
General. Crepidula fornicata is a protandrous hermaphrodite. This means that the animals start their lives as males and then subsequently may change sex and develop into females. Although breeding can occur between February and October, peak activity occurs in May and June when 80-90% of females spawn. Most females spawn twice in a year, apparently after neap tides. The spat settle in isolation or on top of an established chain. If the individual settles alone, it becomes male briefly, passing rapidly on to a female, especially if another animal settles on it to initiate chain formation. Sex change can only occur to the bottom-most male in a stack and takes approximately 60 days, during which the penis regresses and the pouches and glands of the female duct develop. If a juvenile settles on an established stack it develops and may remain as a male for an extended period (up to six years), apparently maintained by pheromones released by females lower in the stack (Fretter & Graham, 1981).
Age at maturity. Due to the protandrous hermaphroditic lifecycle of Crepidula fornicata, age at maturity is difficult to ascertain. Warne(1956), cited in Fretter & Graham (1981), reported size at maturity to be 4mm but it is unclear whether this refers to both sexes or males only. A size of 4 mm would be achieved approximately two months after settlement. Under laboratory conditions, Nelson et al.(1983) report that the mean time from being spawned to first larval release for females was 300 days, i.e.. maturity is reached approximately 10 months after settlement.
Generation time. Generation time is complicated by the hermaphroditic life-cycle of Crepidula fornicata. Incubation of the eggs takes 2-4 weeks and the duration of the larval phase is four to five weeks (Fretter & Graham, 1981; Thouzeau, 1991). Using the ages at maturity quoted above, it would appear that males are capable of breeding as little as four months after fertilization. Under laboratory conditions, Nelson et al. (1983) reported the female generation time to be 300 days. However, in situ females were not reported to spawn until their third year (Deslou-Paoli & Heral, 1986).
Fecundity. Females can lay around 11000 eggs at a time contained in up to 50 egg capsules (Deslou-Paoli & Heral, 1986). Laboratory experiments by Thain (1984) revealed that approximately 4000 larvae per female were released following incubation.
Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceStacks of adult Crepidula fornicata live attached to the substratum and are incapable of moving. Removal of the substratum would remove the resident population and intolerance is therefore recorded as high. Ismail (1985) demonstrated that following suction dredging of the top few centimetres of sediment on oyster grounds in Delaware Bay the Crepidula fornicata population was removed. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | High | High | Moderate | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceStacks of adult Crepidula fornicata live attached to the substratum and are incapable of moving. They are active suspension feeders, generating a water current through the mantle cavity by ciliary action and trapping food particles on a mucous sheet lying across the front surface of the gill filament. Smothering with a 5cm layer of sediment would be expected to clog the feeding and respiration structures. However, it has been demonstrated that Crepidula fornicata is capable of clearing its feeding structures at some energetic cost (Johnson, 1972). Furthermore, areas with large Crepidula fornicata populations do tend to become silted up through deposition of pseudofaeces, apparently with little effect on the species (Thouzeau et al., 2000) and the fact that Crepidula fornicata lives in chains of up to 12 individuals means that at least some of the chain would avoid the effects of smothering. Therefore, although there may be some energetic cost as a result of smothering, probably resulting in decreased growth and reproductive output, there is unlikely to be mortality and an intolerance result of low is recorded. Following the smothering event, growth and reproduction should quickly return to normal and hence a recoverability of very high is recorded. | Low | Very high | Very Low | Low |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceCrepidula fornicata is an active suspension feeder, trapping food particles on a mucous sheet lying across the front surface of the gill filament. An increase in suspended sediment is therefore likely to interfere with the feeding and respiration structures. Johnson (1972) transplanted individual Crepidula fornicata to environments of varying turbidity and measured their shell growth rates. Growth rate was found to decrease as turbidity increased. These observations were verified in laboratory conditions by measuring water filtration rate at different turbidities. Filtration rate was found to decrease as turbidity increased with the greatest reduction in filtration occurring between 140-200 mg l-1. Decreased filtration rate was associated with increased production of pseudofaeces in order to keep the filtering mechanism clear of debris. Increased pseudofaecal production coupled with decreased food intake would lead to increased energy consumption which is likely to impair the survival of the species. Hence, intolerance is recorded as low. When suspended sediment returns to normal levels, feeding and respiration will return to normal and the only likely lag will be in reproductive output, i.e. it will take a period of time to replenish food reserves, during which reproductive output will not be at maximum levels. A recoverability of very high is therefore recorded. | Low | Very high | Very Low | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceCrepidula fornicata is an active suspension feeder, feeding on phytoplankton and particulate organic food. A decrease in suspended sediment would decrease food availability and therefore may impair growth rates. However, over a one month period (the benchmark) it is unlikely that survival would be affected. Hence, intolerance is recorded as low. When turbidity returns to normal levels, growth rate should soon return to normal and hence recoverability is recorded as very high. | Low | Very high | Very Low | Moderate |
Desiccation [Show more]Desiccation
EvidenceThe majority of the population of Crepidula fornicata inhabit the subtidal zone and therefore desiccation is not relevant. However, some individuals are found at the level of low water spring tides and are therefore likely to be intolerant of desiccation. Crepidula fornicata has the ability to attach firmly to its substratum and is thus completely enclosed within its shell. The benchmark for desiccation is exposure to the air for one hour. It is likely that Crepidula fornicata would be able to survive this exposure with only some loss of water. During the period of exposure it would not be able to feed and respiration would be compromised and so there is likely to be some energetic cost. Intolerance is therefore recorded as low. However, it should be noted that Crepidula fornicata does not colonize the intertidal zone and therefore must be prevented from doing so by some factor or combination of factors. This may be desiccation, but it seems more likely to be temperature stress and/or wave exposure. On immersion, metabolic activity should soon return to normal so recoverability is recorded as very high. | Low | Very high | Very Low | Very low |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceCrepidula fornicata is found at the level of low water spring tides and so an increase in emergence will expose some individuals to desiccation stress. The benchmark is an additional one hour of emergence every tidal cycle. During this time, exposed individuals will not be able to feed and respiration will be compromised. Over the period of a year, the resultant energetic cost may cause the mortality of individuals exposed for the longest time. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Low |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceCrepidula fornicata thrives in the subtidal and so individuals on the lower shore which are only exposed rarely anyway would not be affected by a decrease in emergence. It is possible that a decreased emergence regime would result in Crepidula fornicata colonizing further up the shore. | Tolerant | Not relevant | Not sensitive | High |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceCrepidula fornicata is a cosmopolitan species but is found in greatest numbers in wave protected areas (Blanchard, 1997) where water flow is typically moderately strong or weaker (see glossary). An increase in water flow rate of 2 categories for one year would place some individuals in areas of strong or very strong flow. These areas are outside the species' habitat preferences and some mortality is likely, probably due to interference with feeding and/or respiration, although this is not well understood. Intermediate intolerance is therefore recorded. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Low |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceCrepidula fornicata thrives in areas of low water flow (Blanchard, 1997) including the lowest category on the water flow scale ("very weak"). It is an active suspension feeder and is able to maintain a feeding and respiration current independently of ambient water flow. Thus, it would probably tolerate decreases in water flow regime. However, it should be noted that decreased water flow rate could result in deoxygenation and increased settlement of suspended sediment. These factors are covered in their relevant sections. | Tolerant | Not relevant | Not sensitive | High |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceCrepidula fornicata is distributed over a wide temperature range. On the east coast of the Americas it is found as far south as Mexico and therefore it must be able to tolerate higher temperatures than it experiences in northern Europe. The effect of temperature on larval development was investigated by Lucas & Costlow (1979). Larvae were found to tolerate daily temperature cycles of 5°C between 15°C and 30°C with little mortality. Over a 12 day period there was 0% mortality at 30°C but 100% mortality occurred by day 6 at 35°C. Thus, it seems that adults are able to tolerate chronic change over time and larvae are able to tolerate acute change in the short term. Intolerance is therefore recorded as low. The ability of larvae to tolerate acute change also contributes to the species' very high recoverability. | Low | Very high | Very Low | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceDuring the severe winter of 1962-63 the British population of Crepidula fornicata was subjected to an acute decrease in temperatures. Waugh (1964) recorded 25% mortality of Crepidula fornicata from the south coast and east coast of England where the recorded temperatures were 5-6°C and 3-4°C respectively below normal for a period of 2 months. These temperature changes are in line with the benchmarks and hence the intolerance is recorded as intermediate. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | High |
Increase in turbidity [Show more]Increase in turbidity
EvidenceCrepidula fornicata does not require light and therefore is not directly affected by an increase in turbidity for the purposes of light attenuation. The consequent effects that increasing the suspended sediment may have on clogging feeding and respiration structures is covered in 'suspended sediments'. An increase in turbidity may affect primary production in the water column and therefore reduce the availability of phytoplankton food. However, phytoplankton will also immigrate from distant areas and so the effect may be decreased. As the turbidity increase only persists for a year, decreased food availability would probably only affect growth and fecundity and an intolerance of low is recorded. As soon as light levels return to normal, primary production will increase and hence recoverability is recorded as very high. | Low | Very high | Very Low | Low |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceCrepidula fornicata does not require light and therefore is not directly affected by a decrease in turbidity for the purposes of light attenuation. The consequent effects that decreasing the suspended sediment may have on feeding is covered in 'suspended sediments'. | Tolerant | Not relevant | Not sensitive | High |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceCrepidula fornicata is found in greatest numbers in wave protected areas such as bays, estuaries or sheltered sides of wave exposed islands (Blanchard, 1997). This suggests that it is intolerant in some way to wave exposure. The species' body form is robust and it attaches firmly to the substratum but in many cases the substratum is liable to be moved by strong wave action. Indeed, Crepidula fornicata is often found cast ashore following storms (Hayward & Ryland, 1997; F. Viard, pers. comm.). It is also likely that strong wave action will interfere with feeding and/or respiration, although this is poorly understood. A change of 2 categories on the wave exposure scale would place some of the population in the wave exposed category and it is expected that after a year, high mortality would result due to the considerations discussed above, hence an intolerance of high is recorded. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | High | High | Moderate | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceCrepidula fornicata is found in greatest numbers in wave protected areas such as bays, estuaries or sheltered sides of wave exposed islands (Blanchard, 1997). It is an active suspension feeder and is able to maintain a feeding and respiration current independently of ambient water flow. Thus, it would be likely to tolerate decreases in wave exposure. | Tolerant | Not relevant | Not sensitive | High |
Noise [Show more]Noise
EvidenceThere is no evidence to suggest that Crepidula fornicata is sensitive to noise or vibrations caused by noise. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceThere is no evidence to suggest that Crepidula fornicata is sensitive to visual disturbance. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceCrepidula fornicata has a robust body form and so individuals are likely to be resistant to the benchmark level of physical abrasion. However, the gregarious chain-forming characteristic of the species renders it susceptible to disturbance as chains are more likely to be broken up, leaving some individuals exposed to predation. Some mortality is expected and hence intolerance is recorded as intermediate. Due to its high reproductive potential (see additional information below), recoverability is recorded as high.De Montaudouin et al. (2001) (following Sauriau et al., 1998) suggested that physical disturbance is a factor which could stimulate the presence of Crepidula fornicata. They noted that the species settles preferentially in the trails of trawl fishing gear, and that this may explain why Crepidula fornicata is not very abundant in the Arcachon Basin, France, as bottom trawling activities are prohibited here. | Intermediate | High | Low | Very low |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceCrepidula fornicata live in chains of up to 12 individuals, with the bottom individual being attached permanently to the substrate. Attachment is permanent as the shell takes on the shape of the substrate (Hoagland, 1979). Displacement would almost certainly lead to the mortality of the bottom individual in the chain as it would become very vulnerable to predation. However, other individuals in the chain would be unaffected by the displacement. Johnson (1972) demonstrated that transplanted individuals continue to grow normally. The low level of mortality suggests that intolerance to displacement is intermediate. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Moderate |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceNo evidence was found on the effects of synthetic compounds specifically on Crepidula fornicata. However, there is a wealth of evidence concerning effects on related molluscs. The effect of tri-butyl tin (TBT) from anti-fouling paints on gastropods is very well documented. Imposex, female mortality and the subsequent decline in population, has been described in Nucella lapillus (e.g. Bryan et al., 1986), Littorina littorea (Bauer et al., 1995), Ilyanassa obsoleta and Urosalpinx cinerea (Matthiessen & Gibbs, 1998). Limpets (Patellidae) are extremely intolerant of aromatic solvent based dispersants used in oil spill clean-up. During the clean-up response to the Torrey Canyon oil spill nearly all the limpets were killed in areas close to dispersant spraying. Viscous oil will not be readily drawn in under the edge of the shell by ciliary currents in the mantle cavity, whereas detergent, alone or diluted in sea water, would creep in much more readily and be liable to kill the limpet (Smith, 1968). For example, a concentration of 5ppm of dispersant killed half the limpets tested in 24 hours (Southward & Southward, 1978; Hawkins & Southward, 1992). Thus, although no evidence has been found specifically relating to Crepidula fornicata, the intolerance of species in the same class to synthetic chemicals suggests an intolerance of high with moderate confidence. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | High | High | Moderate | Low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceBryan (1984) suggested that gastropods are rather tolerant of heavy metals. In the Fal Estuary, Crepidula fornicata does occur in the Carrick Roads, an area where creek water polluted with heavy metals mixes with the open ocean (Bryan & Gibbs, 1983). In this area, concentrations of silver, cadmium, copper, lead and zinc were found to be higher than in 'control' estuaries (Bryan & Gibbs, 1983). This suggests that Crepidula fornicata is at least partially tolerant to heavy metal contamination. Laboratory trials have revealed specific responses to heavy metals. Thain (1984) investigated the effects of exposure to mercury. The adult and larval 96 hour LC50s (concentrations at which half the organisms die after 96 hours) were 330 and 60 µg l-1 respectively. As a reference, levels of mercury in UK waters at the time of these experiments were 104 to 105 below the 96 hour LC50 for adult Crepidula fornicata. Furthermore, sub-lethal concentrations of mercury were shown to impair growth and condition of young adult Crepidula fornicata and impair reproductive capacity at 0.25µg l-1. Nelson et al. (1983) investigated the effects of exposure to silver. Reproductive output was found to be impaired following exposure to the highest concentration of silver nitrate (10µg l-1) for 24 months. The evidence suggests that high concentrations of heavy metals will cause mortality in Crepidula fornicata and therefore intolerance is recorded as intermediate. Lower concentrations, which could realistically occur in situ impair growth, condition and reproductive output and will therefore affect the long term health of the population. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Moderate |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceNo evidence could be found for the effect of hydrocarbons on Crepidula fornicata specifically. However, inferences can be drawn from closely related groups. Following the Torrey Canyon oil spill in 1967, total mortality of 3 Patella species was reported after one month of oil coming ashore at Porthleven reef (Smith, 1968). Other gastropod mortalities included Nucella lapillus, Tritia incrassata and Gibbula sp.. Bayne et al. (1982) reported that sublethal concentrations of petroleum hydrocarbons depressed the rate of feeding in gastropods and bivalves and increased the rates of oxygen consumption. Increased energy expenditure coupled with decreased feeding rates results in less energy available for growth and reproduction and has been demonstrated to translate to reduced growth rates in juveniles of the bivalve Mercenaria mercenaria (Keck et al., 1978). The lethal effects on gastropods of exposure to high levels of hydrocarbons and the sub-lethal effects of chronic exposure to lower levels suggest that Crepidula fornicata is likely to have a high intolerance to hydrocarbons. However, account has to be taken of the fact that the majority of the population is subtidal and hence is unlikely to be affected by hydrocarbon pollutants. Therefore, the recorded intolerance is adjusted to intermediate. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Low |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceGreenberber et al. (1986) exposed larval Crepidula fornicata to doses of x-ray radiation between 500 and 20,000 rads in total. After 20 days, there was a dose dependent decrease in larval shell growth rate and a significant increase in larval mortality following doses above 2000 rads. These levels of radiation are extremely high compared to background levels in the environment. For reference, Polykarpov (1998) (cited in Cole et al., 1999) describes the natural levels of background radiation being equivalent to a dose of 0.005 Gy per year (equivalent to 0.5 rads per year). Hence, high doses of radiation have been shown to significantly increase mortality while lower levels have sub-lethal effects on growth and reproduction. A value of intermediate intolerance is therefore recorded. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | High |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceInsufficientinformation found. | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidencePopulations of Crepidula fornicata inhabit the open coast where sea water is at full salinity. They are clearly capable of thriving in fully saline conditions and hence probably relatively tolerant of increases in salinity. No information was found concerning the reaction to hypersaline conditions (>40psu). | Tolerant | Not relevant | Not sensitive | High |
Decrease in salinity [Show more]Decrease in salinity
EvidenceCrepidula fornicata is described as euryhaline (Blanchard, 1997) and thrives in variable salinity environments such as estuaries. It would therefore be expected to be tolerant to changes in salinity. However, it is a marine organism and a drop in salinity to levels below 18 psu would be likely to cause water balance stress and therefore impair growth and reproduction. Hence, an intolerance of low is recorded. Recovery is likely to be rapid so a recoverability of very high is recorded. | Low | Very high | Very Low | Moderate |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceCrepidula fornicata is an aerobic organism and therefore will be intolerant in some degree to lack of oxygen. No evidence was found for specific effects of reduced oxygenation on Crepidula fornicata but inferences can be drawn from the effects on other species. Jorgensen (1980) recorded the effects of low oxygen levels on benthic fauna in a Danish fjord. At dissolved oxygen concentrations of 0.2-1.0 mg/l the gastropod Hydrobia ulvae suffered mortality unless able to crawl to areas of higher oxygen concentration and the bivalves, Cardium edule and Mya arenaria, suffered mortality between 2 and 7 days. As Crepidula fornicata is not mobile, it is expected that some mortality would occur within a week at 2 mg/l and an intolerance of intermediate is recorded. Due to its high reproductive potential (see additional information below), recoverability is recorded as high. | Intermediate | High | Low | Very low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo evidence was found concerning microbial infections of Crepidula fornicata. Pecenik et al. (2001) reported that Crepidula fornicata is not infected by trematode miracidia, while other gastropod species including Littorina littorea and Illyanassa obsoleta are highly intolerant. The gastropod ectoparasites Odostomia bisuturalis and Odostomia seminuda both parasitize Crepidula fornicata and the intestinal copepod parasite Mytilicola orientalis has been found in specimens of Crepidula fornicata from Puget Sound, USA, (Kinne, 1980) but there is no evidence concerning the effects. In view of the species' resistance to trematode infection, intolerance is assessed as low. | Low | Very high | Very Low | Moderate |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceCrepidula fornicata is itself an introduced species and has spread widely in Britain since its introduction at the end of the 19th century (see 'Distribution'). | Tolerant | Not relevant | Not sensitive | High |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceCrepidula fornicata is a serious pest on oyster beds (Fretter & Graham, 1981) and therefore extraction of the species has occurred in an attempt to reduce the negative impact on the shellfishery. Cole & Hancock (1956) reported that over 8 tonnes ha-1 of slipper limpets were removed from oyster beds by dredging and that it takes up to 10 years to return to pre-clearance levels. Intolerance is therefore recorded as intermediate and recovery as moderate. | Intermediate | Moderate | Moderate | High |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceCrepidula fornicata is often found attached to other commercially extracted shellfish, particularly oysters (Fretter & Graham, 1981). When the oyster catch is sorted, individuals of Crepidula fornicata are separated from the catch and thrown overboard. De Montaudouin et al. (2001) suggested that this process may favour the proliferation and dispersal of Crepidula fornicata, particularly at the expense of commercially extracted shellfish species. Crepidula fornicata is therefore assessed as not sensitive with the potential to proliferate. | Tolerant* | Not relevant | Not sensitive* | Low |
Additional information
The mode of reproduction of Crepidula fornicata gives the species strong powers of recoverability. Adults spawn at least once a year, large numbers of eggs are produced, there is a long planktotrophic larval stage giving the species high dispersal potential and adults reach maturity within a year. The ability of Crepidula fornicata to colonize new areas has been demonstrated by its spread through Europe following introduction from North America at the end of the 19th century. In light of the above information, recoverability is recorded as high.Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | Non-native |
| Origin | Eastern Canada, North and Central Mexico, Northern America E |
| Date Arrived | 1872 |
Importance information
Ecological impact. High densities of Crepidula fornicata may modify the nature and texture of sediments in some bays (e.g. Ehrhold et al., 1998). Where Crepidula fornicata stacks are abundant, few other bivalves can live amongst them. This is due to spatial competition, trophic competition and alteration of the substratum (the pseudofaeces of Crepidula fornicata smother other bivalves and render the substratum unsuitable for larval settlement) (Fretter & Graham, 1981; Blanchard, 1997). In this way, Crepidula fornicata has become a serious pest on oyster beds and has caused many traditional oyster fisheries to be abandoned (e.g. in the Norman Gulf, France) (Blanchard, 1997). However, there is no indisputable evidence that Crepidula fornicata competes trophically with other species (F. Viard, pers. comm.). De Montaudouin et al. (1999) showed that Crepidula fornicata had no major influence on the local density or diversity of smaller coexisting macroinvertebrates and did not affect the growth of 18-month-old oysters.
Management. In response to the invasion of shellfisheries by Crepidula fornicata, some management has been attempted. Sauriau et al. (1998) and Cole & Hancock (1956) reported dredging operations to clear slipper limpets from oyster beds but concluded that further spread of the species could not be prevented.
Bibliography
Barnes, R.S.K., Coughlan, J. & Holmes, N.J., 1973. A preliminary survey of the macroscopic bottom fauna of the Solent, with particular reference to Crepidula fornicata and Ostrea edulis. Proceedings of the Malacological Society, 40, 253-275.
Bauer, B., Fioroni, P., Ide, I., Liebe, S., Oehlmann, J., Stroben, E. & Watermann, B., 1995. TBT effects on the female genital system of Littorina littorea: a possible indicator of tributyl tin pollution. Hydrobiologia, 309, 15-27.
Bayne, B.L., Widdows, J., Moore, M.N., Salkeld, P., Worrall, C.M. & Donkin, P., 1982. Some ecological consequences of the physiological and biochemical effects of petroleum compounds on marine molluscs. Philosophical Transactions of the Royal Society of London B, 297, 219-239.
Blanchard, M., 1997. Spread of the slipper limpet Crepidula fornicata (L.1758) in Europe. Current state and consequences. Scientia Marina, 61, Supplement 9, 109-118. Available from: http://scimar.icm.csic.es/scimar/index.php/secId/6/IdArt/290/
Bryan, G.W. & Gibbs, P.E., 1983. Heavy metals from the Fal estuary, Cornwall: a study of long-term contamination by mining waste and its effects on estuarine organisms. Plymouth: Marine Biological Association of the United Kingdom. [Occasional Publication, no. 2.]
Bryan, G.W., 1984. Pollution due to heavy metals and their compounds. In Marine Ecology: A Comprehensive, Integrated Treatise on Life in the Oceans and Coastal Waters, vol. 5. Ocean Management, part 3, (ed. O. Kinne), pp.1289-1431. New York: John Wiley & Sons.
Bryan, G.W., Gibbs, P.E., Hummerstone, L.G. & Burt, G.R., 1986. The decline of the gastropod Nucella lapillus around south west England : evidence for the effect of tri-butyl tin from anti-fouling paints. Journal of the Marine Biological Association of the United Kingdom, 66, 611-640.
Cole, H.A. & Hancock, D.A., 1956. Progress in oyster research in Britain 1949-1954, with special reference to the control of pests and diseases. Rapports du Conseils International Pour L'Exploration de la Mer, 140, 24-29.
Connor, D.W., Dalkin, M.J., Hill, T.O., Holt, R.H.F. & Sanderson, W.G., 1997a. Marine biotope classification for Britain and Ireland. Vol. 2. Sublittoral biotopes. Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06., Joint Nature Conservation Committee, Peterborough, JNCC Report no. 230, Version 97.06.
De Montaudoüin, X., Labarraque, D., Giraud, K. & Bachelet, G., 2001. Why does the introduced gastropod Crepidula fornicata fail to invade Arcachon Bay (France)? Journal of the Marine Biological Association of the United Kingdom, 81 (1), 97-104. DOI https://doi.org/10.1017/S0025315401003447
De Montaudouin, X. & Sauriau, P.G., 1999. The proliferating Gastropoda Crepidula fornicata may stimulate macrozoobenthic diversity. Journal of the Marine Biological Association of the United Kingdom, 79, 1069-1077. DOI https://doi.org/10.1017/S0025315499001319
De Montaudouin, X., Andemard, C. & Labourg, P-J., 1999. Does the slipper limpet (Crepidula fornicata L.) impair oyster growth and zoobenthos diversity ? A revisited hypothesis. Journal of Experimental Marine Biology and Ecology, 235, 105-124.
Deslou-Paoli, J.M. & Heral, M., 1986. Crepidula fornicata (L.) (Gastropoda, Calyptraeidae) in the bay of Marennes-Oleron: Biochemical composition and energy value of individuals and spawning. Oceanologica Acta, 9, 305-311.
Ehrhold, A., Blanchard, M., Auffret, J.P. & Garlan, T., 1998. The role of Crepidula proliferation in the modification of the sedimentary tidal environment in Mont Saint-Michel Bay (the Channel, France). Comptes Rendus de L'Acadamie des Sciences. Paris, 327, 583-588.
Eno, N.C., Clark, R.A. & Sanderson, W.G. (ed.) 1997. Non-native marine species in British waters: a review and directory. Peterborough: Joint Nature Conservation Committee.
Fish, J.D. & Fish, S., 1996. A student's guide to the seashore. Cambridge: Cambridge University Press.
Fretter, V. & Graham, A., 1981. The Prosobranch Molluscs of Britain and Denmark. Part 6. Molluscs of Britain and Denmark. Part 6. Journal of Molluscan Studies, Supplement 9, 309-313.
Greenberber, J.S., Pechenik, J.A., Lord, A., Gould, L., Naparstek, E., Kase, K. & Fitzgerald, T.J., 1986. X-irradiation effects on growth and metamorphosis of gastropod larvae (Crepidula fornicata) : a model for environmental radiation teratogenesis. Archives of Environmental Contamination and Toxicology, 15, 227-234.
Hawkins, S.J. & Southward, A.J., 1992. The Torrey Canyon oil spill: recovery of rocky shore communities. In Restoring the Nations Marine Environment, (ed. G.W. Thorpe), Chapter 13, pp. 583-631. Maryland, USA: Maryland Sea Grant College.
Hayward, P., Nelson-Smith, T. & Shields, C. 1996. Collins pocket guide. Sea shore of Britain and northern Europe. London: HarperCollins.
Hayward, P.J. & Ryland, J.S. (ed.) 1995b. Handbook of the marine fauna of North-West Europe. Oxford: Oxford University Press.
Hoagland, K.E., 1979. The behaviour of three sympatric species of Crepidula (Gastropoda : Prosobranchia) from the Atlantic, with implications for evolutionary ecology. Nautilus, 93, 143-149.
Howson, C.M. & Picton, B.E., 1997. The species directory of the marine fauna and flora of the British Isles and surrounding seas. Belfast: Ulster Museum. [Ulster Museum publication, no. 276.]
Ismail, N.S., 1985. The effects of hydraulic dredging to control oyster drills on benthic macrofauna of oyster grounds in Delaware Bay, New Jersey. Internationale Revue der Gesamten Hydrobiologie, 70, 379-395.
JNCC (Joint Nature Conservation Committee), 1999. Marine Environment Resource Mapping And Information Database (MERMAID): Marine Nature Conservation Review Survey Database. [on-line] http://www.jncc.gov.uk/mermaid
Johnson, J.K., 1972. Effect of turbidity on the rate of filtration and growth of the slipper limpet, Crepidula fornicata. Veliger, 14, 315-320.
Jorgensen, B.B., 1980. Seasonal oxygen depletion in the bottom waters of a Danish fjord and its effect on the benthic community. Oikos, 32, 68-76.
Keck, R.T., Hees, R.C., Wehmiller, J. & Maurer, D., 1978. Sublethal effects of the water soluble fraction of Nigerian crude oil on juvenile hard clams, Mercenaria mercenaria (L.). Environmental Pollution, 15, 109-119.
Kinne, O. (ed.), 1980. Diseases of marine animals. vol. 1. General aspects. Protozoa to Gastropoda. Chichester: John Wiley & Sons.
Lucas, J.S. & Costlow J.D., 1979. Effects of various temperature cycles on the larval development of the gastropod mollusc Crepidula fornicata. Marine Biology, 51, 111-117.
Matthiessen, P. & Gibbs, P.E., 1998. Critical appraisal of the evidence for tri-butyl tin mediated endocrine disruption in molluscs. Environmental Toxicology and Chemistry, 17, 37-43.
Minchin, D., McGrath, D. & Duggan, C.B., 1995. The slipper limpet Crepidula fornicata (L.) in Irish waters with a review of its occurrence in the north east Atlantic. Journal of Conchology, 35, 247-254.
Nelson, D.A., Calabrese, A., Greig, R.A., Yevich, P.P. & Chang, S., 1983. Long term silver effects on the marine gastropod Crepidula fornicata. Marine Ecology Progress Series, 12, 155-165.
Pechenik, J.A., Fried, B. & Simpkins, H.L., 2001. Crepidula fornicata is not a first intermediate host for trematodes: who is ? Journal of Experimental Marine Biology and Ecology, 261, 211-224.
Pechenik, J.A., Hilbish, T.J., Eyster, L.S. & Marshall, D., 1996. Relationship between larval and juvenile growth rates in two marine gastropods, Crepidula plana and C. fornicata. Marine Biology, 125, 119-127.
Sauriau, P.G., Pichocki-Seyfried, C., Walker, P., De Montauduin, A., Pascual, A. & Heral, M., 1998. Crepidula fornicata L. (Mollusca, Gastropoda) in the Marennes-Oleron Bay : side-scan sonar mapping of subtidal and stock assessment. Oceanologica Acta, 21, 353-362.
Smith, J.E. (ed.), 1968. 'Torrey Canyon'. Pollution and marine life. Cambridge: Cambridge University Press.
Southward, A.J. & Southward, E.C., 1978. Recolonisation of rocky shores in Cornwall after use of toxic dispersants to clean up the Torrey Canyon spill. Journal of the Fisheries Research Board of Canada, 35, 682-706.
Thain, J.E., 1984. Effects of mercury on the prosobranch mollusc Crepidula fornicata : acute lethal toxicity and effects on growth and reproduction of chronic exposure. Marine Environmental Research, 12, 285-309.
Thouzeau, G., 1991. Experimental collection of postlarvae of Pecten maximus (L.) and other benthic macrofaunal species in the Bay of Saint-Brieuc, France. 1. Reproduction and post larval growth of five mollusc species. Journal of Experimental Marine Biology and Ecology, 148, 181-200.
Thouzeau, G., Chavaud, L., Grall, J. & Guerin, L., 2000. Do biotic interactions control pre-recruitment and growth of Pecten maximus (L.) in the Bay of Brest ? Comptes rendus - acadamies des sciences, Paris, 323, 815-825.
Waugh, G.D., 1964. Effect of severe winter of 1962-63 on oysters and the associated fauna of oyster grounds of southern England. Journal of Animal Ecology, 33, 173-175.
Datasets
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2018. Mollusc (marine) data for Great Britain and Ireland - restricted access. Occurrence dataset: https://doi.org/10.15468/4bsawx accessed via GBIF.org on 2018-09-25.
Conchological Society of Great Britain & Ireland, 2023. Mollusc (marine) records for Great Britain and Ireland. Occurrence dataset: https://doi.org/10.15468/aurwcz accessed via GBIF.org on 2024-09-27.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Isle of Wight Local Records Centre, 2017. Isle of Wight non-native invasive species. Occurrence dataset:https://doi.org/10.15468/laa1k8 accessed via GBIF.org on 2018-09-27.
Kent Wildlife Trust, 2018. Biological survey of the intertidal chalk reefs between Folkestone Warren and Kingsdown, Kent 2009-2011. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Kent Wildlife Trust, 2018. Kent Wildlife Trust Shoresearch Intertidal Survey 2004 onwards. Occurrence dataset: https://www.kentwildlifetrust.org.uk/ accessed via NBNAtlas.org on 2018-10-01.
Merseyside BioBank., 2018. Merseyside BioBank Active Naturalists (unverified). Occurrence dataset: https://doi.org/10.15468/smzyqf accessed via GBIF.org on 2018-10-01.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
Nature Locator, 2017. Sealife Tracker. Occurrence dataset: https://doi.org/10.15468/qgk3pg accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
Norfolk Biodiversity Information Service, 2017. NBIS Records to December 2016. Occurrence dataset: https://doi.org/10.15468/jca5lo accessed via GBIF.org on 2018-10-01.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-08-08
South East Wales Biodiversity Records Centre, 2018. INNS Data: All Taxa (South East Wales). Occurrence dataset: https://doi.org/10.15468/crhjs2 accessed via GBIF.org on 2018-10-02.
South East Wales Biodiversity Records Centre, 2018. SEWBReC Molluscs (South East Wales). Occurrence dataset: https://doi.org/10.15468/jos5ga accessed via GBIF.org on 2018-10-02.
Suffolk Biodiversity Information Service., 2017. Suffolk Biodiversity Information Service (SBIS) Dataset. Occurrence dataset: https://doi.org/10.15468/ab4vwo accessed via GBIF.org on 2018-10-02.
Citation
This review can be cited as:
Last Updated: 17/04/2008