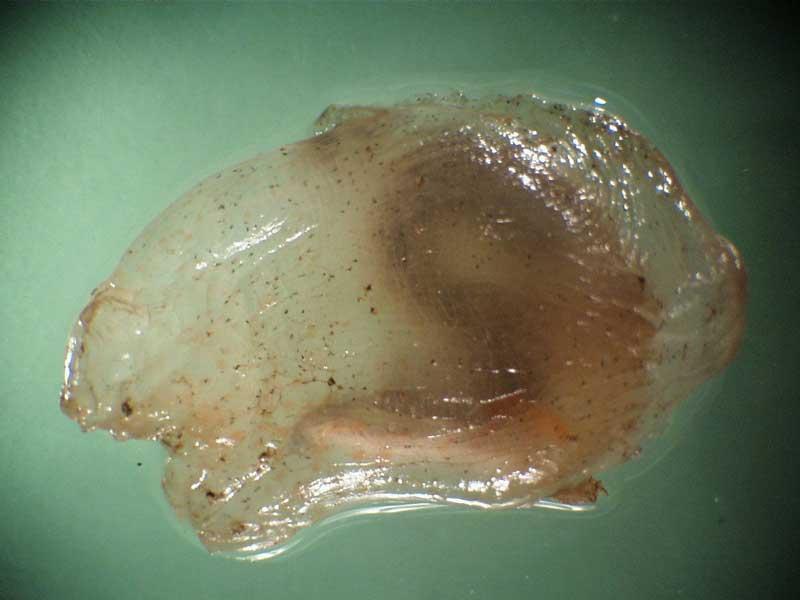
A sea squirt (Ascidiella scabra)
Distribution data supplied by the Ocean Biodiversity Information System (OBIS). To interrogate UK data visit the NBN Atlas.Map Help
| Researched by | Dr Keith Hiscock | Refereed by | This information is not refereed |
| Authority | (Müller, 1776) | ||
| Other common names | - | Synonyms | - |
Summary
Description
Ascidiella scabra is a small solitary ascidian (usually <4cm long) with an ovate body and anterior siphons separated by a distance about one quarter the body length. The test is semi-transparent and usually tinged red.
Recorded distribution in Britain and Ireland
Present all around Britain and Ireland.Global distribution
Present from the Faeroe Islands and Trondheimfjord in the north, occurring in the Kattegat and extending into the Mediterranean.Habitat
Present attached to natural and artificial hard substrata. Also present attached to algae such as Fucus serratus and on kelp stipes. Lindsay and Thompson (1930) suggested a depth range of 5-300 m although records from fucoid algae indicate intertidal occurrence.Depth range
+2-300 mIdentifying features
- Solitary but may occur in tightly packed groups.
- Body ovate or elliptical up to 4 cm long.
- Siphons anterior and separated from each other by about one quarter the body length.
- Test semi-transparent and usually tinged red.
- The internal structure includes 30-110 tentacles, the number always exceeding the number of inner longitudinal vessels of the branchial sac.
Additional information
Specimens as large as 7.5 cm have been sampled from the Dogger Bank. Almost colourless examples can be found.
Listed by
- none -
Biology review
Taxonomy
| Level | Scientific name | Common name |
|---|---|---|
| Phylum | Chordata | Sea squirts, fish, reptiles, birds and mammals |
| Class | Ascidiacea | Sea squirts |
| Order | Phlebobranchia | |
| Family | Ascidiidae | |
| Genus | Ascidiella | |
| Authority | (Müller, 1776) | |
| Recent Synonyms | ||
Biology
| Parameter | Data | ||
|---|---|---|---|
| Typical abundance | Moderate density | ||
| Male size range | <4 cm | ||
| Male size at maturity | |||
| Female size range | Small-medium(3-10cm) | ||
| Female size at maturity | |||
| Growth form | Bullate / Saccate | ||
| Growth rate | |||
| Body flexibility | |||
| Mobility | |||
| Characteristic feeding method | Active suspension feeder, Non-feeding | ||
| Diet/food source | |||
| Typically feeds on | Suspended particles including phytoplankton | ||
| Sociability | |||
| Environmental position | Epibenthic | ||
| Dependency | Independent. | ||
| Supports | None | ||
| Is the species harmful? | No | ||
Biology information
Ascidiella scabra is usually about 2-3 cm in length although specimens from the Dogger Bank have been recorded at 7.5 cm in length (Lindsay & Thompson, 1930)
Habitat preferences
| Parameter | Data |
|---|---|
| Physiographic preferences | Open coast, Offshore seabed, Strait or Sound, Sea loch or Sea lough, Ria or Voe, Estuary, Enclosed coast or Embayment |
| Biological zone preferences | Lower circalittoral, Lower infralittoral, Sublittoral fringe, Upper circalittoral, Upper infralittoral |
| Substratum / habitat preferences | Macroalgae, Artificial (man-made), Bedrock, Biogenic reef, Cobbles, Large to very large boulders, Small boulders, Under boulders |
| Tidal strength preferences | Moderately strong 1 to 3 knots (0.5-1.5 m/sec.), Strong 3 to 6 knots (1.5-3 m/sec.), Weak < 1 knot (<0.5 m/sec.) |
| Wave exposure preferences | Extremely sheltered, Moderately exposed, Sheltered, Very sheltered |
| Salinity preferences | Full (30-40 psu), Variable (18-40 psu) |
| Depth range | +2-300 m |
| Other preferences | No text entered |
| Migration Pattern | Non-migratory or resident |
Habitat Information
-
Life history
Adult characteristics
| Parameter | Data |
|---|---|
| Reproductive type | |
| Reproductive frequency | Annual protracted |
| Fecundity (number of eggs) | |
| Generation time | <1 year |
| Age at maturity | Not known. Probably <6months. |
| Season | March - Insufficient information |
| Life span | 2-5 years |
Larval characteristics
| Parameter | Data |
|---|---|
| Larval/propagule type | - |
| Larval/juvenile development | |
| Duration of larval stage | 2-10 days |
| Larval dispersal potential | 1 km -10 km |
| Larval settlement period |
Life history information
Lindsay & Thompson (1930) noted the great fecundity of Ascidiella scabra and that eggs were produced (in the laboratory) from March onwards. Berrill (1950) notes that the species is oviparous, that the eggs are small (0.16 mm diameter) and sink in still water. Tadpole larvae emerge from eggs.Sensitivity review
The MarLIN sensitivity assessment approach used below has been superseded by the MarESA (Marine Evidence-based Sensitivity Assessment) approach (see menu). The MarLIN approach was used for assessments from 1999-2010. The MarESA approach reflects the recent conservation imperatives and terminology and is used for sensitivity assessments from 2014 onwards.
Physical pressures
Use / to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Substratum loss [Show more]Substratum lossBenchmark. All of the substratum occupied by the species or biotope under consideration is removed. A single event is assumed for sensitivity assessment. Once the activity or event has stopped (or between regular events) suitable substratum remains or is deposited. Species or community recovery assumes that the substratum within the habitat preferences of the original species or community is present. Further details EvidenceThe species is permanently attached to the substratum so substratum loss will result in loss of the population. Therefore an intolerance of high has been reported. For recoverability, see additional information below. | High | Very high | Low | High |
Smothering [Show more]SmotheringBenchmark. All of the population of a species or an area of a biotope is smothered by sediment to a depth of 5 cm above the substratum for one month. Impermeable materials, such as concrete, oil, or tar, are likely to have a greater effect. Further details. EvidenceThe species is permanently attached to the substratum and is an active suspension feeder so that some clearance of smothering silt may occur. The species can extend its siphons to a small extent above silt. It can also most likely maintain a passage through the silt to the siphons.Ascidiella scabra also attaches to other erect biota and, in such situations, may escape smothering effects. Intolerance is likely to be low. Recovery of condition is likely to be very high. | Low | Immediate | Not sensitive | Moderate |
Increase in suspended sediment [Show more]Increase in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceAscidiella scabra frequently occurs in habitats with high levels of suspended matter. Robbins (1985b) undertook experiments to establish the possible effects of high inorganic particulate concentrations on Ascidiella scabra. He concluded that growth rate was likely to be reduced and mortality was possible in high levels of suspended sediment. Therefore an intolerance of low has been recorded. On resumption of normal conditions, energy expenditure and feeding should be restored rapidly. | Low | Immediate | Not sensitive | Moderate |
Decrease in suspended sediment [Show more]Decrease in suspended sedimentBenchmark. An arbitrary short-term, acute change in background suspended sediment concentration e.g., a change of 100 mg/l for one month. The resultant light attenuation effects are addressed under turbidity, and the effects of rapid settling out of suspended sediment are addressed under smothering. Further details EvidenceAlthough there may be some reliance on the organic material associated with suspended silt for nutrition, the reduced need for energy expenditure to remove silt may be beneficial. On balance, the species is most likely tolerant. | Tolerant | Not relevant | Not sensitive | Moderate |
Desiccation [Show more]Desiccation
EvidenceThe species occurs in the intertidal near to low water level and so is exposed to some desiccation. Nevertheless, it has a soft body and may be easily subject to drying-up. Exposure to desiccating influences for one hour will probably kill a proportion of the population. Therefore, an intolerance of intermediate has been recorded. For recoverability, see additional information below. | Intermediate | Very high | Low | Moderate |
Increase in emergence regime [Show more]Increase in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceThe species occurs in the intertidal near to low water level and so is exposed to some emergence. Nevertheless, it has a soft body and may be easily subject to drying-up. Exposure to desiccating influences as a result of increased emergence will probably kill a proportion of the population. Therefore, an intolerance of intermediate has been recorded. For recoverability, see additional information below. | Intermediate | Very high | Low | Moderate |
Decrease in emergence regime [Show more]Decrease in emergence regimeBenchmark. A one hour change in the time covered or not covered by the sea for a period of one year. Further details EvidenceAs a predominantly sublittoral species, increase in emergence may benefit populations found on the lower shore by providing additional substratum for colonization. | Tolerant* | Not relevant | Not sensitive* | High |
Increase in water flow rate [Show more]Increase in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAs a general rule, ascidians require a reasonable water flow rate in order to ensure sufficient food availability. High water flow rates may also be detrimental to feeding ability and posture. Hiscock (1983) found that, for the solitary ascidian Ascidia mentula, siphons closed when the current velocity rose above about 15 cm/sec. It seems likely therefore that some reduction in feeding would occur with increased water flow rate although that would result in slower growth and loss of condition but not mortality. Intolerance has therefore been assessed as low. On resumption of normal energy expenditure and feeding, condition should be restored rapidly. | Low | Immediate | Not sensitive | High |
Decrease in water flow rate [Show more]Decrease in water flow rateA change of two categories in water flow rate (view glossary) for 1 year, for example, from moderately strong (1-3 knots) to very weak (negligible). Further details EvidenceAs a general rule, ascidians require a reasonable water flow rate in order to ensure sufficient food availability and oxygen supply. However, ascidians are active suspension feeders and can thrive in conditions of very little flow. Whilst food availability may be reduced in comparison with areas with higher flow rates, on resumption of normal energy expenditure and feeding, condition should be restored rapidly. | Low | Immediate | Not sensitive | Moderate |
Increase in temperature [Show more]Increase in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceIn the North Atlantic and Mediterranean where Ascidiella scabra occurs, temperatures may be higher by several degrees than in Britain and Ireland. It is not therefore expected that increased temperatures at the level of the benchmark will adversely affect populations. | Tolerant | Not relevant | Not sensitive | High |
Decrease in temperature [Show more]Decrease in temperature
For intertidal species or communities, the range of temperatures includes the air temperature regime for that species or community. Further details EvidenceAscidiella scabra occurs north to Trondheim in Norway and the Faroe Islands, where temperatures may be lower by several degrees than in Britain and Ireland. Crisp (1964) indicates that no certain mortality was observed in ascidians following the severe 1962-63 winter. It is not expected therefore that decreased temperatures at the level of the benchmark will adversely affect populations. | Tolerant | Not relevant | Not sensitive | High |
Increase in turbidity [Show more]Increase in turbidity
EvidenceAscidiella scabra lives in estuaries and other enclosed areas where turbidity may increase to high levels. It is not expected that increase in turbidity at the level of the benchmark will adversely affect Ascidiella scabra. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in turbidity [Show more]Decrease in turbidity
EvidenceAlthough there may be some reliance on the organic material associated with turbidity for nutrition, the reduced need for energy expenditure to clear any silt that may be causing turbidity may be beneficial and an intolerance of tolerant* has been recorded. | Tolerant* | Not relevant | Not sensitive* | Moderate |
Increase in wave exposure [Show more]Increase in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceAs a general rule, ascidians require a reasonable water flow rate in order to ensure sufficient food availability and oxygen supply. However, high water flow rates may be detrimental to feeding ability and posture. Hiscock (1983) found that, for the solitary ascidian Ascidia mentula, siphons closed when current velocity rose above about 15 cm/sec. It seems likely therefore that some reduction in feeding would occur with increased oscillatory water movement although that would result in slower growth and loss of condition but not mortality. On resumption of normal energy expenditure and feeding, condition should be restored rapidly. Although individuals are firmly attached, there is a possibility that wave action may displace large numbers. Intermediate intolerance but with low confidence is recorded. Recovery is likely to be very high (see additional information below). | Intermediate | Very high | Low | Low |
Decrease in wave exposure [Show more]Decrease in wave exposureA change of two ranks on the wave exposure scale (view glossary) e.g., from Exposed to Extremely exposed for a period of one year. Further details EvidenceAs a general rule, ascidians require a reasonable water flow rate in order to ensure sufficient food availability and oxygen supply and maintain surfaces clean of silt. If decrease in wave action occurs where tidal flow continues to provide favourable conditions, the species may benefit because of reduction in the likelihood of displacement. Whilst food availability may be reduced by reduction in wave action, on resumption of normal energy expenditure and feeding, condition should be restored rapidly. Overall, bearing in mind that the favoured location for Ascidiella scabra is in wave sheltered habitats, the species might benefit from decrease in wave exposure. | Tolerant* | Not relevant | Not sensitive* | High |
Noise [Show more]Noise
EvidenceTunicates are not known to have organs sensitive to noise. | Tolerant | Not relevant | Not sensitive | High |
Visual presence [Show more]Visual presenceBenchmark. The continuous presence for one month of moving objects not naturally found in the marine environment (e.g., boats, machinery, and humans) within the visual envelope of the species or community under consideration. Further details EvidenceTunicates are not known to respond to visual presence. | Tolerant | Not relevant | Not sensitive | High |
Abrasion & physical disturbance [Show more]Abrasion & physical disturbanceBenchmark. Force equivalent to a standard scallop dredge landing on or being dragged across the organism. A single event is assumed for assessment. This factor includes mechanical interference, crushing, physical blows against, or rubbing and erosion of the organism or habitat of interest. Where trampling is relevant, the evidence and trampling intensity will be reported in the rationale. Further details. EvidenceEpifaunal species have been found to be particularly adversely affected by trawling or dredging activities, either due to direct damage or modification of the substratum (Jennings & Kaiser, 1998). However, some epifaunal species have been reported to exhibit increased abundances on high fishing effort areas, probably due to their ability to colonize and grow rapidly (Bradshaw et al., 2000). In a study of the long term effects of scallop dredging, Bradshaw et al. (2002) reported that Ascidiella species had become more abundant and suggested that they were probably able to survive by regeneration of damage and budding. Individuals are easily ripped from the substratum and are unlikely to re-attach and will die. Intolerance is therefore high. For recoverability, see additional information. | High | Very high | Low | High |
Displacement [Show more]DisplacementBenchmark. Removal of the organism from the substratum and displacement from its original position onto a suitable substratum. A single event is assumed for assessment. Further details EvidenceThe colonies are attached permanently to the substratum and will not re-attach so that displacement, even if to a suitable habitat, would most likely result in mortality. An assessment of high intolerance is therefore made. For recoverability, see additional information below. | High | Very high | Low | High |
Chemical pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Synthetic compound contamination [Show more]Synthetic compound contaminationSensitivity is assessed against the available evidence for the effects of contaminants on the species (or closely related species at low confidence) or community of interest. For example:
The evidence used is stated in the rationale. Where the assessment can be based on a known activity then this is stated. The tolerance to contaminants of species of interest will be included in the rationale when available; together with relevant supporting material. Further details. EvidenceAscidians may be intolerant of synthetic chemicals such as tri-butyl-tin anti-foulants. Rees et al. (2001), working in the Crouch estuary, observed that six ascidian species were recorded at one station in 1997 compared with only two at the same station in 1987, shortly following the banning of TBT in antifouling paints. Also, there was a marked increase in the abundance of ascidians especially Ascidiella aspersa and Ascidia conchilega in the estuary. No evidence has been found for sublethal effects from which recovery would be likely to be rapid. Overall, an intolerance of intermediate is suggested but with a low confidence. | Intermediate | Very high | Low | Low |
Heavy metal contamination [Show more]Heavy metal contaminationEvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Hydrocarbon contamination [Show more]Hydrocarbon contaminationEvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Radionuclide contamination [Show more]Radionuclide contaminationEvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Changes in nutrient levels [Show more]Changes in nutrient levelsEvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Increase in salinity [Show more]Increase in salinity
EvidenceAscidiella scabra occurs in full salinity although it may be abundant in variable salinity or reduced salinity (for instance in the biotope ECR.HbowEud (Halichondria (Halichondria) bowerbanki, Eudendrium arbusculum and Eucratea loricata on reduced salinity tide-swept circalittoral mixed substrata). Therefore, it is not expected that increase in salinity will have an adverse effect except in the possibility of allowing other species to out-complete Ascidiella scabra. | Tolerant | Not relevant | Not sensitive | Moderate |
Decrease in salinity [Show more]Decrease in salinity
EvidenceA fall in salinity from full to reduced would not be expected to have an adverse effect as Ascidiella scabra occurs in reduced salinity conditions. However, in situations where salinity is already variable or reduced, a further lowering is likely to result in mortality. Intolerance is indicated as intermediate but may be high. For recoverability, see additional information. | Intermediate | Very high | Low | Moderate |
Changes in oxygenation [Show more]Changes in oxygenationBenchmark. Exposure to a dissolved oxygen concentration of 2 mg/l for one week. Further details. EvidenceAscidians are active suspension feeders that pump water. It seems likely that the effects of lowered oxygenation will be reduced as stagnation can be avoided. An intolerance of low is therefore suggested but with very low confidence. Recovery is likely to be immediate. | Low | Immediate | Not sensitive | Very low |
Biological pressures
Use [show more] / [show less] to open/close text displayed
| Intolerance | Recoverability | Sensitivity | Evidence / Confidence | |
Introduction of microbial pathogens/parasites [Show more]Introduction of microbial pathogens/parasitesBenchmark. Sensitivity can only be assessed relative to a known, named disease, likely to cause partial loss of a species population or community. Further details. EvidenceNo information has been found. | No information | Not relevant | No information | Not relevant |
Introduction of non-native species [Show more]Introduction of non-native speciesSensitivity assessed against the likely effect of the introduction of alien or non-native species in Britain or Ireland. Further details. EvidenceThere are no non-native species currently known to displace or adversely affect Ascidiella scabra although the ascidian Perophora japonica may occur in similar habitats. | Tolerant | Not relevant | Not sensitive | Moderate |
Extraction of this species [Show more]Extraction of this speciesBenchmark. Extraction removes 50% of the species or community from the area under consideration. Sensitivity will be assessed as 'intermediate'. The habitat remains intact or recovers rapidly. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThere is no known extraction of this species. | Not relevant | Not relevant | Not relevant | Not relevant |
Extraction of other species [Show more]Extraction of other speciesBenchmark. A species that is a required host or prey for the species under consideration (and assuming that no alternative host exists) or a keystone species in a biotope is removed. Any effects of the extraction process on the habitat itself are addressed under other factors, e.g. displacement, abrasion and physical disturbance, and substratum loss. Further details. EvidenceThere are no species with which Ascidiella scabra is associated that may be extracted. | Not relevant | Not relevant | Not relevant | Not relevant |
Additional information
Ascidiella scabra has a high fecundity and settles readily, probably for an extended period from spring to autumn. Svane (1988) describes it as "an annual ascidian" and demonstrated recruitment onto artificial and scraped natural substrata. Eggs and larvae are free-living for only a few hours and so recolonization would have to be from existing individuals no more than a few km away. It is also likely that Ascidiella scabra larvae are attracted by existing populations and settle near to adults (Svane et al., 1987) . Fast growth means that a dense cover could be established within about 2 months. However, if mortality and the consequent establishment of free space available occurs at a time when larvae are not being produced, other species may settle and dominate. Therefore a recoverability of 'very high' is for when larvae are available to settle. If another species colonizes and dominates the substratum, recolonization by Ascidiella scabra may take several years.Importance review
Policy/legislation
- no data -
Status
| National (GB) importance | - | Global red list (IUCN) category | - |
Non-native
| Parameter | Data |
|---|---|
| Native | - |
| Origin | - |
| Date Arrived | - |
Importance information
Ascidiella scabra is a fast colonizing species and may be a fouling organism.Bibliography
Berrill, N.J., 1950. The Tunicata with an account of the British species. London: Ray Society.
Bradshaw, C., Veale, L.O., Hill, A.S. & Brand, A.R., 2002. The role of scallop-dredge disturbance in long-term changes in Irish Sea benthic communities: a re-analysis of an historical dataset. Journal of Sea Research, 47, 161-184. DOI https://doi.org/10.1016/S1385-1101(02)00096-5
Crisp, D.J. (ed.), 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology, 33, 165-210.
Hiscock, K., 1983. Water movement. In Sublittoral ecology. The ecology of shallow sublittoral benthos (ed. R. Earll & D.G. Erwin), pp. 58-96. Oxford: Clarendon Press.
Lindsay, S.J. & Thompson, H. 1930. The determination of specific characters for the identification of certain ascidians. Journal of the Marine Biological Association of the United Kingdom, 17, 1-35.
Millar, R.H., 1970. British Ascidians London: Academic Press.[Synopses of the British Fauna, no. 1.]
Rees, H.L., Waldock, R., Matthiessen, P. & Pendle, M.A., 2001. Improvements in the epifauna of the Crouch estuary (United Kingdom) following a decline in TBT concentrations. Marine Pollution Bulletin, 42, 137-144. DOI https://doi.org/10.1016/S0025-326X(00)00119-3
Robbins, I.J. 1985b. Ascidian growth rate and survival at high inorganic particulate concentrations. Marine Pollution Bulletin, 16, 365-367.
Svane, I, Havenhund, J.N. & Jorgensen, A.J., 1987. Effects of tissue extract of adults on metamorphosis in Ascidia mentula O.F. Mueller and Ascidiella scabra (O.F. Müller). Journal of Experimental Marine Biology and Ecology, 110, 171-181.
Svane, I., 1988. Recruitment and development of epibioses on artificial and cleared substrata at two site in Gullmarsfjorden on the Swedish west coast. Ophelia, 29, 25-41.
Datasets
Centre for Environmental Data and Recording, 2018. IBIS Project Data. Occurrence dataset: https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Centre for Environmental Data and Recording, 2018. Ulster Museum Marine Surveys of Northern Ireland Coastal Waters. Occurrence dataset https://www.nmni.com/CEDaR/CEDaR-Centre-for-Environmental-Data-and-Recording.aspx accessed via NBNAtlas.org on 2018-09-25.
Fenwick, 2018. Aphotomarine. Occurrence dataset http://www.aphotomarine.com/index.html Accessed via NBNAtlas.org on 2018-10-01
Manx Biological Recording Partnership, 2022. Isle of Man historical wildlife records 1990 to 1994. Occurrence dataset:https://doi.org/10.15468/aru16v accessed via GBIF.org on 2024-09-27.
National Trust, 2017. National Trust Species Records. Occurrence dataset: https://doi.org/10.15468/opc6g1 accessed via GBIF.org on 2018-10-01.
NBN (National Biodiversity Network) Atlas. Available from: https://www.nbnatlas.org.
OBIS (Ocean Biodiversity Information System), 2025. Global map of species distribution using gridded data. Available from: Ocean Biogeographic Information System. www.iobis.org. Accessed: 2025-07-30
Outer Hebrides Biological Recording, 2018. Invertebrates (except insects), Outer Hebrides. Occurrence dataset: https://doi.org/10.15468/hpavud accessed via GBIF.org on 2018-10-01.
South East Wales Biodiversity Records Centre, 2023. SEWBReC Marine and other Aquatic Invertebrates (South East Wales). Occurrence dataset:https://doi.org/10.15468/zxy1n6 accessed via GBIF.org on 2024-09-27.
Citation
This review can be cited as:
Last Updated: 24/04/2006